Current Organic Synthesis ( IF 1.7 ) Pub Date : 2021-11-30 , DOI: 10.2174/1570179418666210614142939 Laishram M Devi 1 , Thokchom P Singh 1 , Okram M Singh 1
|
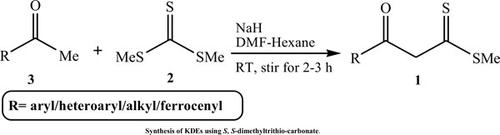
β-Ketodithioesters (KDEs) are versatile building blocks for the rapid construction of various heterocyclic compounds. Quite a good number of successful reactions based on KDEs have been developed in the past decade for the construction of heterocyclic skeletons under mild conditions. This review covers the new CC/ X bond formation and annulation reactions of KDEs with dielectrophilic or dinucleophilic reagents. Multicomponent reactions using KDEs to construct various heterocycles are also the major contents in this review.
Objective: The aim of this review is to bring a fresh perspective on the application of KDEs in organic synthesis covering from 2013 to 2020.
Conclusion: From this review, it has been cleared that KDEs have been the object of numerous studies on its use in heterocyclic synthesis. The presence of different functional groups on this synthon permits the incorporation of C-C/X sources into the final targets, which is the significant property of KDEs.
中文翻译:

β-酮二硫酯的CC/杂原子键形成和环化反应的最新进展
β-酮二硫酯 (KDE) 是用于快速构建各种杂环化合物的通用构件。在过去的十年中,已经开发了大量基于 KDE 的成功反应,用于在温和条件下构建杂环骨架。本综述涵盖了 KDE 与介电或双亲核试剂的新 CC/X 键形成和环化反应。利用 KDE 构建各种杂环的多组分反应也是本综述的主要内容。
目的:本综述的目的是为 2013 年至 2020 年 KDE 在有机合成中的应用带来新的视角。
结论:从这篇综述中可以清楚地看出,KDE 已成为许多关于其在杂环合成中的应用研究的对象。该合成器上不同官能团的存在允许将 CC/X 源合并到最终目标中,这是 KDE 的重要特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号