Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-07-17 , DOI: 10.1016/j.seppur.2021.119203 Yueping Bao 1 , Wen Jie Lee 1, 2 , Chaoting Guan 3 , Yen Nan Liang 1 , Teik-Thye Lim 4 , Xiao Hu 1, 5
|
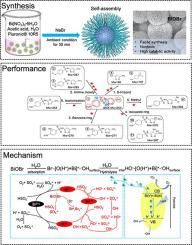
A 3D flower-like bismuth oxybromide (BiOBr) was synthesized by a facile one-pot chemical precipitation method and its potential on sulfamethoxazole (SMX) degradation via peroxymonosulfate (PMS) activation was investigated for the first time. The physic-chemical properties of BiOBr were characterized by field emission scanning electron microscope (FESEM), high resolution transmission electron microscope (HRTEM), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The key influencing factors in SMX degradation (including catalyst loading, PMS dosage, pH etc.) were discussed. The attack sites and degradation pathway were proposed via the identification of the reactive sites on SMX molecular together with the detection of intermediate degradation products. Furthermore, the toxicity of the intermediate products was evaluated via the ecological activity relationship (ECOSAR) program and the PMS activation mechanism over BiOBr was proposed via the determination of reactive species. Hydroxyl radical and singlet oxygen were identified as the main reactive species in the system and the enhanced catalytic efficiency of BiOBr was attributed to the active metal (Bi3+-Bi5+-Bi3+) redox cycles as well as the light effect. The enhancement on SMX degradation from light effect could be observed under visible light irradiation, even at ambient light condition. Finally, the robustness of the BiOBr/PMS system was further examined via multiple organics degradation as well as the performance comparison with other metal oxides. These findings provide an insight for BiOBr as a viable and superior alternative for PMS activation in organics degradation.
中文翻译:

环境条件下溴化铋高效活化过氧单硫酸盐以降解磺胺甲恶唑:合成、性能、动力学和机制
通过简便的一锅化学沉淀法合成了 3D 花状溴氧化铋 (BiOBr),并首次研究了其通过过硫酸盐 (PMS) 活化降解磺胺甲恶唑 (SMX) 的潜力。通过场发射扫描电子显微镜(FESEM)、高分辨透射电子显微镜(HRTEM)、X射线衍射(XRD)和X射线光电子能谱(XPS)对BiOBr的理化性质进行了表征。讨论了影响 SMX 降解的关键因素(包括催化剂负载量、PMS 用量、pH 值等)。通过识别SMX分子上的反应位点并检测中间降解产物,提出了攻击位点和降解途径。此外,通过生态活动关系(ECOSAR)程序评估中间产物的毒性,并通过确定活性物种提出了BiOBr的PMS活化机制。羟基自由基和单线态氧被确定为系统中的主要反应物种,BiOBr 催化效率的提高归因于活性金属(Bi3+ -Bi 5+ -Bi 3+ ) 氧化还原循环以及光效应。即使在环境光条件下,在可见光照射下也可以观察到光效应对 SMX 降解的增强。最后,通过多种有机物降解以及与其他金属氧化物的性能比较,进一步检验了 BiOBr/PMS 系统的稳健性。这些发现为 BiOBr 作为有机物降解中 PMS 活化的可行且优越的替代方案提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号