当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of a novel naphthalenone endoperoxide and structural elucidation by nuclear magnetic resonance spectroscopy and theoretical calculation
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2021-07-15 , DOI: 10.1002/mrc.5195 Lucas M O S Martins 1 , Juliana O Santos 1 , Thomas Hoye 2 , Elson S Alvarenga 1
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2021-07-15 , DOI: 10.1002/mrc.5195 Lucas M O S Martins 1 , Juliana O Santos 1 , Thomas Hoye 2 , Elson S Alvarenga 1
Affiliation
|
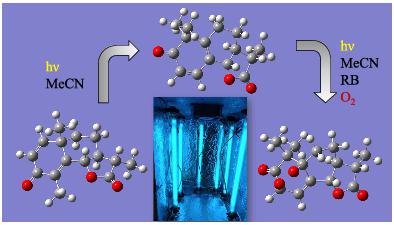
Sesquiterpene lactones are found in plants of Asteraceae family, and endoperoxides are known for their antimalarial activity. Structural elucidation is a relevant aspect; however, it is not uncommon to find incorrect or incomplete structural assignments in the literature. Calculations based in quantum mechanics are frequently used to compute 1H and 13C NMR chemical shifts, and after comparing with the experimental data, the correct structure is established from diverse candidates. Targeting the synthesis of bioactive compounds, we envisaged the synthesis of a novel endoperoxide from the natural sesquiterpene lactone α-santonin (2). Photochemical transformation of α-santonin (2) to mazdasantonin (4) followed by photooxidation catalyzed by rose bengal afforded the novel endoperoxide 5. This new endoperoxide contains five stereogenic centers and is analogous to the antimalarial agent artemisinin (1). The relative configuration of the stereogenic centers of the endoperoxide were established by nuclear magnetic resonance (NMR) analyses and confirmed by theoretical calculations. All approaches were in complete agreement, and the structure of mazdasantonin endoperoxide was established as (3S,3aS,5aS,8R,9bS)-3,6,6-trimethyl-3,3a,4,5,8,9b-hexahydro-2H-5a,8-epidioxynaphtho[1,2-b]furan-2,7(6H)-dione.
中文翻译:

一种新型萘酮内过氧化物的合成及核磁共振波谱和理论计算的结构解析
倍半萜内酯存在于菊科植物中,内过氧化物以其抗疟活性而闻名。结构说明是一个相关的方面;然而,在文献中发现不正确或不完整的结构分配并不罕见。经常使用基于量子力学的计算来计算1 H 和13 C NMR 化学位移,并在与实验数据进行比较后,从不同的候选对象中建立正确的结构。针对生物活性化合物的合成,我们设想从天然倍半萜内酯α- santonin合成新型内过氧化物( 2 )。α-山东宁 ( 2 ) 向马他山东宁 ( ) 的光化学转化4 ) 随后由玫瑰红催化的光氧化得到新型内过氧化物5。这种新的内过氧化物包含五个立体中心,类似于抗疟药青蒿素 ( 1 )。内过氧化物立体中心的相对构型是通过核磁共振 (NMR) 分析建立的,并通过理论计算得到证实。所有方法均完全一致,并确定马他山酮内过氧化物的结构为(3 S ,3a S ,5a S ,8 R ,9b S )-3,6,6-trimethyl-3,3a,4,5,8 , 9b-六氢-2 H -5a,8-epidioxynaphtho[1,2 - b ]furan-2,7(6H )-二酮。
更新日期:2021-07-15
中文翻译:

一种新型萘酮内过氧化物的合成及核磁共振波谱和理论计算的结构解析
倍半萜内酯存在于菊科植物中,内过氧化物以其抗疟活性而闻名。结构说明是一个相关的方面;然而,在文献中发现不正确或不完整的结构分配并不罕见。经常使用基于量子力学的计算来计算1 H 和13 C NMR 化学位移,并在与实验数据进行比较后,从不同的候选对象中建立正确的结构。针对生物活性化合物的合成,我们设想从天然倍半萜内酯α- santonin合成新型内过氧化物( 2 )。α-山东宁 ( 2 ) 向马他山东宁 ( ) 的光化学转化4 ) 随后由玫瑰红催化的光氧化得到新型内过氧化物5。这种新的内过氧化物包含五个立体中心,类似于抗疟药青蒿素 ( 1 )。内过氧化物立体中心的相对构型是通过核磁共振 (NMR) 分析建立的,并通过理论计算得到证实。所有方法均完全一致,并确定马他山酮内过氧化物的结构为(3 S ,3a S ,5a S ,8 R ,9b S )-3,6,6-trimethyl-3,3a,4,5,8 , 9b-六氢-2 H -5a,8-epidioxynaphtho[1,2 - b ]furan-2,7(6H )-二酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号