当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ile209 of Leishmania donovani xanthine phosphoribosyltransferase plays a key role in determining its purine base specificity
FEBS Letters ( IF 3.0 ) Pub Date : 2021-07-16 , DOI: 10.1002/1873-3468.14162 Bhumi Patel 1 , Dhaval Patel 1 , Anju Pappachan 1, 2
FEBS Letters ( IF 3.0 ) Pub Date : 2021-07-16 , DOI: 10.1002/1873-3468.14162 Bhumi Patel 1 , Dhaval Patel 1 , Anju Pappachan 1, 2
Affiliation
|
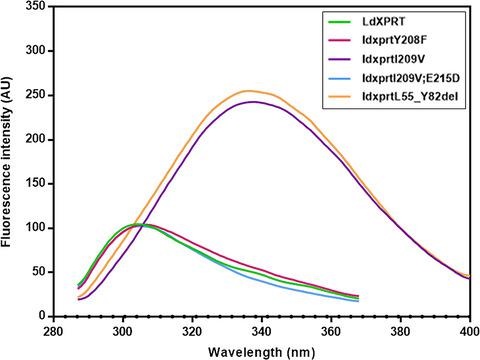
Xanthine phosphoribosyltransferase (XPRT) and hypoxanthine-guanine phosphoribosyltransferase (HGPRT) are purine salvaging enzymes of Leishmania donovani with distinct 6-oxopurine specificities. LdXPRT phosphoribosylates xanthine, hypoxanthine, and guanine, with preference toward xanthine, whereas LdHGPRT phosphoribosylates only hypoxanthine and guanine. In our study, LdXPRT was used as a model to understand these purine base specificities. Mutating I209 to V, the conserved residue found in HGPRTs, reduced the affinity of LdXPRT for xanthine, converting it to an HGXPRT-like enzyme. The Y208F mutation in the active site indicated that aromatic residue interactions with the purine ring are limited to pi-pi binding forces and do not impart purine base specificity. Deleting the unique motif (L55-Y82) of LdXPRT affected enzyme activity. Our studies established I209 as a key residue determining the 6-oxopurine specificity of LdXPRT.
中文翻译:

杜氏利什曼原虫黄嘌呤磷酸核糖转移酶的 Ile209 在决定其嘌呤碱基特异性方面发挥关键作用
黄嘌呤磷酸核糖转移酶 (XPRT) 和次黄嘌呤鸟嘌呤磷酸核糖转移酶 (HGPRT) 是杜氏利什曼原虫的嘌呤回收酶,具有独特的 6-氧代嘌呤特异性。 LdXPRT 磷酸核糖基化黄嘌呤、次黄嘌呤和鸟嘌呤,优先考虑黄嘌呤,而 LdHGPRT 仅磷酸核糖基化次黄嘌呤和鸟嘌呤。在我们的研究中,LdXPRT 被用作了解这些嘌呤碱基特异性的模型。将 I209 突变为 V(HGPRT 中发现的保守残基)会降低 LdXPRT 对黄嘌呤的亲和力,将其转化为 HGXPRT 样酶。活性位点中的 Y208F 突变表明芳香残基与嘌呤环的相互作用仅限于 pi-pi 结合力,并且不赋予嘌呤碱基特异性。删除 LdXPRT 的独特基序 (L55-Y82) 会影响酶活性。我们的研究确定 I209 是决定 LdXPRT 6-氧代嘌呤特异性的关键残基。
更新日期:2021-08-23
中文翻译:

杜氏利什曼原虫黄嘌呤磷酸核糖转移酶的 Ile209 在决定其嘌呤碱基特异性方面发挥关键作用
黄嘌呤磷酸核糖转移酶 (XPRT) 和次黄嘌呤鸟嘌呤磷酸核糖转移酶 (HGPRT) 是杜氏利什曼原虫的嘌呤回收酶,具有独特的 6-氧代嘌呤特异性。 LdXPRT 磷酸核糖基化黄嘌呤、次黄嘌呤和鸟嘌呤,优先考虑黄嘌呤,而 LdHGPRT 仅磷酸核糖基化次黄嘌呤和鸟嘌呤。在我们的研究中,LdXPRT 被用作了解这些嘌呤碱基特异性的模型。将 I209 突变为 V(HGPRT 中发现的保守残基)会降低 LdXPRT 对黄嘌呤的亲和力,将其转化为 HGXPRT 样酶。活性位点中的 Y208F 突变表明芳香残基与嘌呤环的相互作用仅限于 pi-pi 结合力,并且不赋予嘌呤碱基特异性。删除 LdXPRT 的独特基序 (L55-Y82) 会影响酶活性。我们的研究确定 I209 是决定 LdXPRT 6-氧代嘌呤特异性的关键残基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号