Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2021-05-01 , DOI: 10.2174/1570180817999201104125630 Konstantinos M. Kasiotis 1 , George Lambrinidis 2 , Nikolas Fokialakis 3 , Serkos A. Haroutounian 1
|
Background: Tamoxifen (TAM), a non-steroidal antiestrogen, constitutes the endocrine treatment of choice against breast cancer. Since its inauguration, substantial effort has been devoted towards the design and synthesis of TAM’s analogues aiming to improve its bioactivity and reveal their structure-activity relationship.
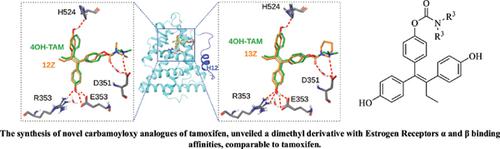
Objective: One of the most studied synthetic features of TAM’s structure is the ether side chain, which is strongly related to its positioning into the active site of the Estrogen Receptors (ERα and ERβ). Herein, we present the application of a straightforward route for the efficient synthesis of selected novel carbamoyloxy analogues of TAM and the evaluation of their respective binding affinities to the Estrogen Receptors α and β.
Methods: A one-pot reaction was applied for the construction of TAM’s triarylethylene core moiety, which subsequently was derivatized to provide efficiently the target carbamoyloxy analogues of TAM. The Z and E isomers of the latter were separated using RP-HPLC-UV and their binding affinities to ERα and ERβ were measured.
Results: Among all compounds synthesized, the dimethyl derivative was determined as the most potent for both receptors, displaying binding affinity values comparable to TAM, though the Zdiethyl analogue maintained substantial affinity to both ERs. The aforementioned results were further studied by theoretical calculations and molecular modelling to delineate a concordance among calculations and biological activity.
Conclusion: Approach applied herein permitted the extraction of a useful structure-activity relationship correlation pattern highlighting the importance of a chemically stabilized tamoxifen side chain.
中文翻译:

他莫昔芬的新型氨基甲酰氧基类似物:合成、分子对接和生物活性评估
背景:他莫昔芬 (TAM) 是一种非甾体抗雌激素,是乳腺癌内分泌治疗的首选。自成立以来,TAM 类似物的设计和合成已投入大量精力,旨在提高其生物活性并揭示其构效关系。
目的:TAM 结构的研究最多的合成特征之一是醚侧链,这与其在雌激素受体(ERα 和 ERβ)的活性位点中的定位密切相关。在这里,我们提出了一种直接途径的应用,用于有效合成选定的新型 TAM 氨基甲酰氧基类似物,并评估它们各自对雌激素受体 α 和 β 的结合亲和力。
方法:采用一锅法构建 TAM 的三芳基乙烯核心部分,随后对其进行衍生化以有效提供 TAM 的目标氨基甲酰氧基类似物。后者的 Z 和 E 异构体使用 RP-HPLC-UV 进行分离,并测量了它们对 ERα 和 ERβ 的结合亲和力。
结果:在合成的所有化合物中,二甲基衍生物被确定为对两种受体最有效,显示出与 TAM 相当的结合亲和力值,尽管 Zdiethyl 类似物对两种 ER 保持显着的亲和力。通过理论计算和分子建模进一步研究了上述结果,以描绘计算和生物活性之间的一致性。
结论:本文应用的方法允许提取有用的构效关系相关模式,突出化学稳定的他莫昔芬侧链的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号