当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Portobelamides A and B and Caciqueamide, Cytotoxic Peptidic Natural Products from a Caldora sp. Marine Cyanobacterium
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-07-16 , DOI: 10.1021/acs.jnatprod.0c01383 Ozlem Demirkiran 1, 2 , Jehad Almaliti 2 , Tiago Leão 2, 3 , Gabriel Navarro 2 , Tara Byrum 2 , Frederick A Valeriote 4 , Lena Gerwick 2 , William H Gerwick 2, 3
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-07-16 , DOI: 10.1021/acs.jnatprod.0c01383 Ozlem Demirkiran 1, 2 , Jehad Almaliti 2 , Tiago Leão 2, 3 , Gabriel Navarro 2 , Tara Byrum 2 , Frederick A Valeriote 4 , Lena Gerwick 2 , William H Gerwick 2, 3
Affiliation
|
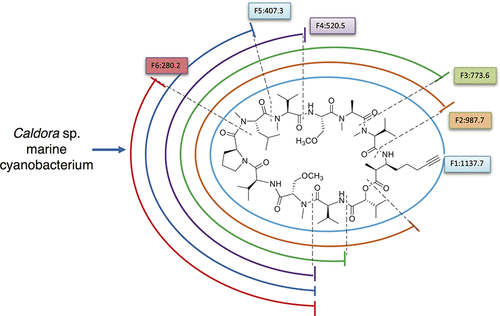
Three new compounds, portobelamides A and B (1 and 2), 3-amino-2-methyl-7-octynoic acid (AMOYA) and hydroxyisovaleric acid (Hiva) containing cyclic depsipeptides, and one long chain lipopeptide caciqueamide (3), were isolated from a field-collection of a Caldora sp. marine cyanobacterium obtained from Panama as part of the Panama International Cooperative Biodiversity Group Program. Their planar structures were elucidated through analysis of 2D NMR and MS data, especially high resolution (HR) MS2/MS3 fragmentation methods. The absolute configurations of compounds 1 and 2 were deduced by traditional hydrolysis, derivative formation, and chromatographic analyses compared with standards. Portobelamide A (1) showed good cytotoxicity against H-460 human lung cancer cells (33% survival at 0.9 μM).
中文翻译:

Portobelamides A 和 B 以及 Caciqueamide,来自 Caldora sp. 的细胞毒性肽天然产物。海洋蓝藻
三种新化合物,portobelamides A 和 B(1和2)、3-氨基-2-甲基-7-辛酸 (AMOYA) 和羟基异戊酸 (Hiva) 含有环状缩肽,以及一种长链脂肽 caciqueamide ( 3 )从Caldora sp的现场收集中分离出来。作为巴拿马国际合作生物多样性小组计划的一部分,从巴拿马获得的海洋蓝藻。通过对 2D NMR 和 MS 数据的分析,特别是高分辨率 (HR) MS 2 /MS 3碎裂方法,阐明了它们的平面结构。化合物1和2的绝对构型通过传统的水解、衍生物的形成和色谱分析与标准品进行比较推断。Portobelamide A ( 1 ) 对 H-460 人肺癌细胞显示出良好的细胞毒性(0.9 μM 时存活率为 33%)。
更新日期:2021-08-27
中文翻译:

Portobelamides A 和 B 以及 Caciqueamide,来自 Caldora sp. 的细胞毒性肽天然产物。海洋蓝藻
三种新化合物,portobelamides A 和 B(1和2)、3-氨基-2-甲基-7-辛酸 (AMOYA) 和羟基异戊酸 (Hiva) 含有环状缩肽,以及一种长链脂肽 caciqueamide ( 3 )从Caldora sp的现场收集中分离出来。作为巴拿马国际合作生物多样性小组计划的一部分,从巴拿马获得的海洋蓝藻。通过对 2D NMR 和 MS 数据的分析,特别是高分辨率 (HR) MS 2 /MS 3碎裂方法,阐明了它们的平面结构。化合物1和2的绝对构型通过传统的水解、衍生物的形成和色谱分析与标准品进行比较推断。Portobelamide A ( 1 ) 对 H-460 人肺癌细胞显示出良好的细胞毒性(0.9 μM 时存活率为 33%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号