当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterologous Catalysis of the Final Steps of Tetracycline Biosynthesis by Saccharomyces cerevisiae
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-07-16 , DOI: 10.1021/acschembio.1c00259 Ehud Herbst 1 , Arden Lee 1 , Yi Tang 2 , Scott A Snyder 3 , Virginia W Cornish 1, 4
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-07-16 , DOI: 10.1021/acschembio.1c00259 Ehud Herbst 1 , Arden Lee 1 , Yi Tang 2 , Scott A Snyder 3 , Virginia W Cornish 1, 4
Affiliation
|
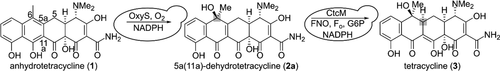
Developing treatments for antibiotic resistant bacterial infections is among the highest priority public health challenges worldwide. Tetracyclines, one of the most important classes of antibiotics, have fallen prey to antibiotic resistance, necessitating the generation of new analogs. Many tetracycline analogs have been accessed through both total synthesis and semisynthesis, but key C-ring tetracycline analogs remain inaccessible. New methods are needed to unlock access to these analogs, and heterologous biosynthesis in a tractable host such as Saccharomyces cerevisiae is a candidate method. C-ring analog biosynthesis can mimic nature’s biosynthesis of tetracyclines from anhydrotetracyclines, but challenges exist, including the absence of the unique cofactor F420 in common heterologous hosts. Toward this goal, this paper describes the biosynthesis of tetracycline from anhydrotetracycline in S. cerevisiae heterologously expressing three enzymes from three bacterial hosts: the anhydrotetracycline hydroxylase OxyS, the dehydrotetracycline reductase CtcM, and the F420 reductase FNO. This biosynthesis of tetracycline is enabled by OxyS performing just one hydroxylation step in S. cerevisiae despite its previous characterization as a double hydroxylase. This single hydroxylation enabled us to purify and structurally characterize a hypothetical intermediate in oxytetracycline biosynthesis that can explain structural differences between oxytetracycline and chlortetracycline. We show that Fo, a synthetically accessible derivative of cofactor F420, can replace F420 in tetracycline biosynthesis. Critically, the use of S. cerevisiae for the final steps of tetracycline biosynthesis described herein sets the stage to achieve a total biosynthesis of tetracycline as well as novel tetracycline analogs in S. cerevisiae with the potential to combat antibiotic-resistant bacteria.
中文翻译:

酿酒酵母生物合成四环素最后步骤的异源催化
开发抗生素耐药性细菌感染的治疗方法是全球最优先的公共卫生挑战之一。四环素是最重要的抗生素类别之一,已成为抗生素耐药性的牺牲品,因此需要产生新的类似物。许多四环素类似物已通过全合成和半合成获得,但关键的 C 环四环素类似物仍然无法获得。需要新的方法来解锁对这些类似物的访问,而在易处理的宿主(如酿酒酵母)中进行异源生物合成是一种候选方法。C 环类似物的生物合成可以模拟自然界从无水四环素合成四环素,但存在挑战,包括缺乏独特的辅因子 F 420在共同的异源宿主中。为了实现这一目标,本文描述了在酿酒酵母中从异源表达来自三种细菌宿主的三种酶的无水四环素生物合成四环素:无水四环素羟化酶 OxyS、脱氢四环素还原酶 CtcM 和 F 420还原酶 FNO。这种四环素的生物合成是通过 OxyS 在酿酒酵母中仅进行一个羟基化步骤实现的,尽管其先前被表征为双羟化酶。这种单一的羟基化使我们能够纯化并在结构上表征土霉素生物合成中的一种假设中间体,该中间体可以解释土霉素和金霉素之间的结构差异。我们证明了 F o,一种可合成的辅因子 F 420衍生物,可在四环素生物合成中替代 F 420 。至关重要的是,将酿酒酵母用于本文所述的四环素生物合成的最后步骤为在酿酒酵母中实现四环素以及新型四环素类似物的总生物合成奠定了基础,具有对抗抗生素抗性细菌的潜力。
更新日期:2021-08-20
中文翻译:

酿酒酵母生物合成四环素最后步骤的异源催化
开发抗生素耐药性细菌感染的治疗方法是全球最优先的公共卫生挑战之一。四环素是最重要的抗生素类别之一,已成为抗生素耐药性的牺牲品,因此需要产生新的类似物。许多四环素类似物已通过全合成和半合成获得,但关键的 C 环四环素类似物仍然无法获得。需要新的方法来解锁对这些类似物的访问,而在易处理的宿主(如酿酒酵母)中进行异源生物合成是一种候选方法。C 环类似物的生物合成可以模拟自然界从无水四环素合成四环素,但存在挑战,包括缺乏独特的辅因子 F 420在共同的异源宿主中。为了实现这一目标,本文描述了在酿酒酵母中从异源表达来自三种细菌宿主的三种酶的无水四环素生物合成四环素:无水四环素羟化酶 OxyS、脱氢四环素还原酶 CtcM 和 F 420还原酶 FNO。这种四环素的生物合成是通过 OxyS 在酿酒酵母中仅进行一个羟基化步骤实现的,尽管其先前被表征为双羟化酶。这种单一的羟基化使我们能够纯化并在结构上表征土霉素生物合成中的一种假设中间体,该中间体可以解释土霉素和金霉素之间的结构差异。我们证明了 F o,一种可合成的辅因子 F 420衍生物,可在四环素生物合成中替代 F 420 。至关重要的是,将酿酒酵母用于本文所述的四环素生物合成的最后步骤为在酿酒酵母中实现四环素以及新型四环素类似物的总生物合成奠定了基础,具有对抗抗生素抗性细菌的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号