当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal Chemistry of the Heavier Alkaline Earth Atoms Ca, Sr, and Ba
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-07-15 , DOI: 10.1021/acs.accounts.1c00277 Mingfei Zhou 1 , Gernot Frenking 2, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-07-15 , DOI: 10.1021/acs.accounts.1c00277 Mingfei Zhou 1 , Gernot Frenking 2, 3
Affiliation
|
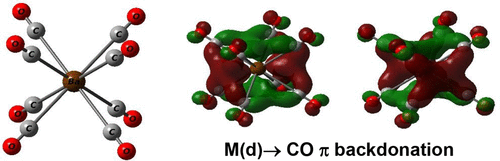
Alkaline earth elements beryllium, magnesium, calcium, strontium, and barium with an ns2 valence-shell configuration are usually classified as main-group elements that belong to the s-block atoms. For a long time, the elements were considered to be rather chemically uninteresting atomic species due to preconceived ideas about bonding, structure, and reactivity. They typically use the two ns valence electrons in forming ionic salt compounds with the metal in a formal oxidation state of +2. For the heavier alkaline earth atoms, calcium, strontium, and barium, their (n – 1)d atomic orbitals (AOs) are empty but lie close in energy to the valence np orbitals. Earlier theoretical investigations have already suggested that these elements can employ the (n – 1)d AOs to some extent to form polar bonds in divalent species in which the alkaline earth metal centers are sufficiently positively charged. The d orbital involvement increases from Ca to Sr and markedly in Ba. Thus, barium has been termed an honorary transition metal.
中文翻译:

较重碱土原子 Ca、Sr 和 Ba 的过渡金属化学
具有n s 2价壳构型的碱土元素铍、镁、钙、锶和钡通常被归类为属于 s 区原子的主族元素。长期以来,由于对键合、结构和反应性的先入为主的想法,这些元素被认为是在化学上相当无趣的原子种类。它们通常使用两个n s 价电子与处于 +2 形式氧化态的金属形成离子盐化合物。对于较重的碱土原子,钙、锶和钡,它们的 ( n – 1)d 原子轨道 (AO) 是空的,但其能量接近于价np 轨道。早期的理论研究已经表明,这些元素可以在一定程度上利用 ( n – 1) d AO 在碱土金属中心带足够正电荷的二价物种中形成极性键。d 轨道参与从 Ca 增加到 Sr,并在 Ba 中显着增加。因此,钡被称为荣誉过渡金属。
更新日期:2021-08-03
中文翻译:

较重碱土原子 Ca、Sr 和 Ba 的过渡金属化学
具有n s 2价壳构型的碱土元素铍、镁、钙、锶和钡通常被归类为属于 s 区原子的主族元素。长期以来,由于对键合、结构和反应性的先入为主的想法,这些元素被认为是在化学上相当无趣的原子种类。它们通常使用两个n s 价电子与处于 +2 形式氧化态的金属形成离子盐化合物。对于较重的碱土原子,钙、锶和钡,它们的 ( n – 1)d 原子轨道 (AO) 是空的,但其能量接近于价np 轨道。早期的理论研究已经表明,这些元素可以在一定程度上利用 ( n – 1) d AO 在碱土金属中心带足够正电荷的二价物种中形成极性键。d 轨道参与从 Ca 增加到 Sr,并在 Ba 中显着增加。因此,钡被称为荣誉过渡金属。











































 京公网安备 11010802027423号
京公网安备 11010802027423号