当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development and Scale-Up of an Asymmetric Synthesis of AZD8186 Using the Fukuyama Modification of the Mitsunobu Reaction
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-07-15 , DOI: 10.1021/acs.oprd.1c00133 Peter R. Moore 1 , James C. Muir 2 , Jerome Dubiez 1 , Kevin W. Leslie 2 , Paula Tomlin 2 , Marc McCormick 1 , Sophie L. Janbon 1 , Philip Cornwall 1 , Per Ryberg 3 , Robert Berg 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-07-15 , DOI: 10.1021/acs.oprd.1c00133 Peter R. Moore 1 , James C. Muir 2 , Jerome Dubiez 1 , Kevin W. Leslie 2 , Paula Tomlin 2 , Marc McCormick 1 , Sophie L. Janbon 1 , Philip Cornwall 1 , Per Ryberg 3 , Robert Berg 3
Affiliation
|
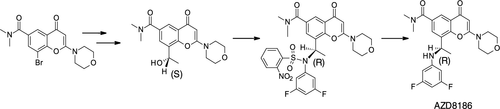
A large-scale asymmetric synthesis has been developed for the kilo-lab manufacture of AZD8186. The process initially employs a regioselective Heck coupling in water to provide the starting aromatic ketone. This ketone is reduced asymmetrically under ruthenium-catalyzed transfer hydrogenation conditions to provide a chiral alcohol in high enantiomeric purity. The key synthetic step then requires the reaction of this chiral alcohol with the activated derivative of 3,5-difluoroaniline under the Mitsunobu reaction conditions. The common issues associated with the use of the Mitsunobu reaction, such as removal of triphenylphosphine oxide and reduced diisopropyl azodicarboxylate (DIAD) by-products, have been eliminated through crystallization of the relevant intermediates.
中文翻译:

AZD8186 不对称合成的开发和放大使用光信反应的福山改性
为 AZD8186 的公斤实验室制造开发了大规模不对称合成。该方法最初在水中采用区域选择性 Heck 偶联来提供起始芳香酮。该酮在钌催化的转移氢化条件下不对称还原,以提供高对映体纯度的手性醇。然后,关键的合成步骤需要这种手性醇与 3,5-二氟苯胺的活化衍生物在光信反应条件下反应。与使用 Mitsunobu 反应相关的常见问题,例如去除氧化三苯基膦和还原偶氮二羧酸二异丙酯 (DIAD) 副产物,已通过相关中间体的结晶消除。
更新日期:2021-08-20
中文翻译:

AZD8186 不对称合成的开发和放大使用光信反应的福山改性
为 AZD8186 的公斤实验室制造开发了大规模不对称合成。该方法最初在水中采用区域选择性 Heck 偶联来提供起始芳香酮。该酮在钌催化的转移氢化条件下不对称还原,以提供高对映体纯度的手性醇。然后,关键的合成步骤需要这种手性醇与 3,5-二氟苯胺的活化衍生物在光信反应条件下反应。与使用 Mitsunobu 反应相关的常见问题,例如去除氧化三苯基膦和还原偶氮二羧酸二异丙酯 (DIAD) 副产物,已通过相关中间体的结晶消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号