当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
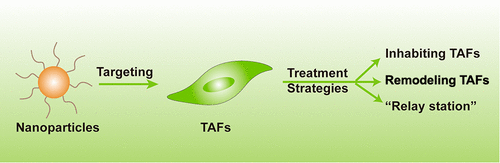
Tumor-Associated Fibroblast-Targeting Nanoparticles for Enhancing Solid Tumor Therapy: Progress and Challenges
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-07-14 , DOI: 10.1021/acs.molpharmaceut.1c00455 Wenpan Li 1 , Nicholas Little 1 , Jonghan Park 1 , Cole Alexander Foster 1 , Jiawei Chen 2 , Jianqin Lu 1, 3, 4, 5
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-07-14 , DOI: 10.1021/acs.molpharmaceut.1c00455 Wenpan Li 1 , Nicholas Little 1 , Jonghan Park 1 , Cole Alexander Foster 1 , Jiawei Chen 2 , Jianqin Lu 1, 3, 4, 5
Affiliation
|
Even though nanoparticle drug delivery systems (nanoDDSs) have improved antitumor efficacy by delivering more drugs to tumor sites compared to free and unencapsulated therapeutics, achieving satisfactory distribution and penetration of nanoDDSs inside solid tumors, especially in stromal fibrous tumors, remains challenging. As one of the most common stromal cells in solid tumors, tumor-associated fibroblasts (TAFs) not only promote tumor growth and metastasis but also reduce the drug delivery efficiency of nanoparticles through the tumor’s inherent physical and physiological barriers. Thus, TAFs have been emerging as attractive targets, and TAF-targeting nanotherapeutics have been extensively explored to enhance the tumor delivery efficiency and efficacy of various anticancer agents. The purpose of this Review is to opportunely summarize the underlying mechanisms of TAFs on obstructing nanoparticle-mediated drug delivery into tumors and discuss the current advances of a plethora of nanotherapeutic approaches for effectively targeting TAFs.
中文翻译:

肿瘤相关成纤维细胞靶向纳米颗粒用于增强实体瘤治疗:进展和挑战
尽管纳米颗粒药物递送系统(nanoDDSs)通过向肿瘤部位递送更多药物来提高抗肿瘤功效,但与游离和未封装的治疗剂相比,实现纳米DDSs在实体瘤内的令人满意的分布和渗透,尤其是在间质纤维瘤中,仍然具有挑战性。作为实体瘤中最常见的基质细胞之一,肿瘤相关成纤维细胞(TAFs)不仅促进了肿瘤的生长和转移,而且通过肿瘤固有的物理和生理屏障降低了纳米颗粒的药物递送效率。因此,TAF 已成为有吸引力的靶标,并且已广泛探索靶向 TAF 的纳米疗法以提高各种抗癌剂的肿瘤递送效率和功效。
更新日期:2021-08-03
中文翻译:

肿瘤相关成纤维细胞靶向纳米颗粒用于增强实体瘤治疗:进展和挑战
尽管纳米颗粒药物递送系统(nanoDDSs)通过向肿瘤部位递送更多药物来提高抗肿瘤功效,但与游离和未封装的治疗剂相比,实现纳米DDSs在实体瘤内的令人满意的分布和渗透,尤其是在间质纤维瘤中,仍然具有挑战性。作为实体瘤中最常见的基质细胞之一,肿瘤相关成纤维细胞(TAFs)不仅促进了肿瘤的生长和转移,而且通过肿瘤固有的物理和生理屏障降低了纳米颗粒的药物递送效率。因此,TAF 已成为有吸引力的靶标,并且已广泛探索靶向 TAF 的纳米疗法以提高各种抗癌剂的肿瘤递送效率和功效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号