Molecular Cell ( IF 14.5 ) Pub Date : 2021-07-15 , DOI: 10.1016/j.molcel.2021.06.025 Adam S B Jalal 1 , Ngat T Tran 1 , Ling J Wu 2 , Karunakaran Ramakrishnan 1 , Martin Rejzek 3 , Giulia Gobbato 1 , Clare E M Stevenson 4 , David M Lawson 4 , Jeff Errington 2 , Tung B K Le 1
|
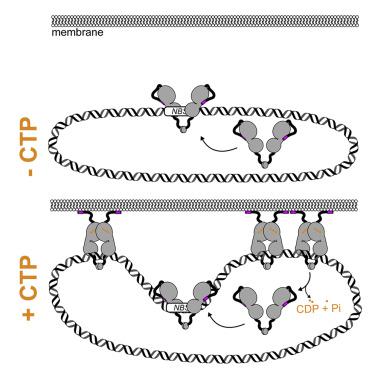
ATP- and GTP-dependent molecular switches are extensively used to control functions of proteins in a wide range of biological processes. However, CTP switches are rarely reported. Here, we report that a nucleoid occlusion protein Noc is a CTPase enzyme whose membrane-binding activity is directly regulated by a CTP switch. In Bacillus subtilis, Noc nucleates on 16 bp NBS sites before associating with neighboring non-specific DNA to form large membrane-associated nucleoprotein complexes to physically occlude assembly of the cell division machinery. By in vitro reconstitution, we show that (1) CTP is required for Noc to form the NBS-dependent nucleoprotein complex, and (2) CTP binding, but not hydrolysis, switches Noc to a membrane-active state. Overall, we suggest that CTP couples membrane-binding activity of Noc to nucleoprotein complex formation to ensure productive recruitment of DNA to the bacterial cell membrane for nucleoid occlusion activity.
中文翻译:

CTP 调节类核封闭蛋白 Noc 的膜结合活性
ATP 和 GTP 依赖性分子开关广泛用于控制蛋白质在各种生物过程中的功能。然而,CTP 开关很少被报道。在这里,我们报告了类核封闭蛋白 Noc 是一种 CTPase 酶,其膜结合活性由 CTP 开关直接调节。在枯草芽孢杆菌中,Noc 在 16 bp NBS位点上成核,然后与相邻的非特异性 DNA 结合形成大的膜相关核蛋白复合物,从而物理阻断细胞分裂机制的组装。通过体外重组,我们表明 (1) Noc 需要 CTP 才能形成NBS依赖的核蛋白复合物和 (2) CTP 结合,但不是水解,将 Noc 切换到膜活性状态。总体而言,我们建议 CTP 将 Noc 的膜结合活性与核蛋白复合物的形成结合起来,以确保将 DNA 有效地募集到细菌细胞膜以实现类核闭塞活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号