Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-07-15 , DOI: 10.1016/j.seppur.2021.119282 Zhi-Heng Lu 1 , Ibrahim Abdelhai Senosy 1 , Dong-Dong Zhou 1 , Zhong-Hua Yang 1 , Hao-Ming Guo 1 , Xiao Liu 1
|
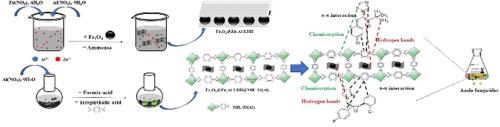
In this work, a novel Fe3O4@ZnAl-LDH@MIL-53(Al) composite was synthesized by a facile three-step process and utilized as an adsorbent for the removal of azole fungicides from environmental water. The characterization results revealed that double hydroxide (ZnAl-LDH) and metal organic framework (MIL-53) have been successfully tethered to the shell of Fe3O4. The main factors affecting the removal efficiencies were investigated, and results revealed that Fe3O4@ZnAl-LDH@MIL-53(Al) composite possessed high removal efficiencies for azole fungicides. Furthermore, the adsorption mechanism was investigated and including hydrogen-bonding interaction, chemisorption and π–π interaction. The adsorption kinetic was more consistent with the pseudo-second-order kinetic model, and the whole adsorption process could reach the equilibrium within 5 min. Langmuir adsorption isotherm model could describe the adsorption process most accurately, and the maximum adsorption values of azole fungicides were in the range of 43.54–71.79 mg g−1. Meanwhile, the adsorption thermodynamics indicated that the adsorption process was exothermic and spontaneous. Hence, Fe3O4@ZnAl-LDH@MIL-53(Al) composite had a great application potential in the removal of azole fungicides from the environment.
中文翻译:

Fe3O4@ZnAl-LDH@MIL-53(Al)用于去除环境水中唑类杀菌剂的合成及吸附性能研究
在这项工作中,通过简单的三步法合成了一种新型的 Fe 3 O 4 @ZnAl-LDH@MIL-53(Al) 复合材料,并将其用作吸附剂从环境水中去除唑类杀菌剂。表征结果表明,双氢氧化物(ZnAl-LDH)和金属有机骨架(MIL-53)已成功地束缚在Fe 3 O 4的壳上。考察了影响去除效率的主要因素,结果表明,Fe 3 O 4@ZnAl-LDH@MIL-53(Al) 复合材料对唑类杀菌剂具有很高的去除效率。此外,研究了吸附机制,包括氢键相互作用、化学吸附和 π-π 相互作用。吸附动力学更符合拟二级动力学模型,整个吸附过程可在5 min内达到平衡。Langmuir吸附等温线模型可以最准确地描述吸附过程,唑类杀菌剂的最大吸附值在43.54-71.79 mg g -1范围内。同时,吸附热力学表明吸附过程是放热自发的。因此,Fe 3 O 4@ZnAl-LDH@MIL-53(Al) 复合材料在去除环境中的唑类杀菌剂方面具有巨大的应用潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号