Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-07-15 , DOI: 10.1016/j.seppur.2021.119309 Jiaxin Tong 1 , Zhiping Zhu 1 , Mingpeng He 1 , Pan Zhou 1 , Yuankang Jiang 1 , Zhenggang Wang 1
|
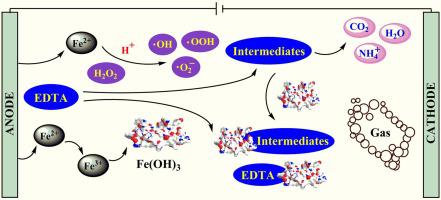
The electrochemical degradation kinetics of cleaning wastewater containing ethylene diamine tetraacetic acid (EDTA) is complicated. In this study, the kinetics of EDTA degradation was conditioned on an experiment device designed for the electrochemical treatment of EDTA cleaning wastewater, and five parameters, namely, initial pH, electrode distance, current density, supporting electrolyte concentration and H2O2 concentration, were investigated. The single-factor experimental results indicated that an acidic initial pH or a small electrode distance is beneficial to electrochemical degradation, the supporting electrolyte concentration is changing the rate for the initial stage of reaction, and high current density or excessive H2O2 concentration could reduce the oxidation efficiency. On the basis of single-factor experiments, three factors that affect the reaction rate significantly were taken as variable factors in the Box–Behnken experimental design (i.e. initial pH (X1 = 3–5), current density (X2 = 40–60 A/m2) and H2O2 concentration (X3 = 20–40 mmol/L)). The optimum condition could be obtained at the initial pH of 4.3, the current density of 53.0 A/m2 and the H2O2 concentration of 37.6 mmol/L, and degradation constants k, k1, k2 and k3 are 0.0247, 0.0134, 0.2077 and 0.0256 min−1, respectively. The SEM and EDX analyses were performed on the precipitate and scum after electrochemical treatment, and a possible degradation mechanism of EDTA was proposed.
中文翻译:

乙二胺四乙酸清洗废水的电化学降解动力学
含有乙二胺四乙酸 (EDTA) 的清洗废水的电化学降解动力学是复杂的。在本研究中,EDTA 降解的动力学取决于为 EDTA 清洗废水的电化学处理设计的实验装置,以及五个参数,即初始 pH 值、电极距离、电流密度、支持电解质浓度和 H 2 O 2浓度,被调查。单因素实验结果表明,酸性初始pH值或小电极间距有利于电化学降解,支持电解质浓度改变反应初始阶段的速率,高电流密度或过量H 2 O 2浓度会降低氧化效率。在单因素实验的基础上,Box-Behnken 实验设计中将三个显着影响反应速率的因素作为可变因素(即初始pH(X 1 = 3-5)、电流密度(X 2 = 40-)。 60 A/m 2 ) 和 H 2 O 2浓度 (X 3 = 20–40 mmol/L))。最佳条件为初始pH 4.3,电流密度53.0 A/m 2,H 2 O 2浓度37.6 mmol/L,降解常数k、k 1、k 2和k 3分别为 0.0247、0.0134、0.2077 和 0.0256 min -1。对电化学处理后的沉淀物和浮渣进行SEM和EDX分析,提出了可能的EDTA降解机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号