International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-07-15 , DOI: 10.1016/j.ijms.2021.116671 Nicole Eyet 1 , Shaun G. Ard 2 , Nicholas S. Shuman 2 , Albert A. Viggiano 2
|
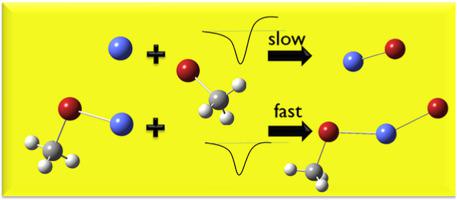
The temperature- and pressure-dependent kinetics of Co+(CH3Br)n (n = 0,1) + CH3Br are measured from 300 to 600 K and from 0.2 to 0.5 Torr. Results are interpreted using density functional calculations and modeled with statistical theory. In the n = 0 case, the associative product Co+(CH3Br) dominates with a rate constant between 5 and 30% of the collisional value, while a minor channel yields CoBr+ + CH3 with a rate constant increasing with temperature. 0K bond dissociation energies (BDE) are determined for Co+-Br (2.8 ± 0.1 eV) and Co+-CH3Br (2.4 ± 0.25 eV). Ligation of Co+ (i.e. n = 1) significantly increases the bimolecular reaction rate constant. CoBr+(CH3Br) + CH3 is formed and the reaction has a positive temperature dependence. Interestingly, this rate constant decreases with increasing pressure due to competition of the association channel. Ligation increases the (CH3Br)Co+-Br BDE (3.0 ± 0.1 eV) relative to Co+-Br, but decreases the (CH3Br)Co+-CH3Br BDE (1.95 ± 0.25) relative to Co+-CH3Br. Both bimolecular reactions proceed by a metal-insertion mechanism with a significantly submerged transition state that does not affect the kinetics. Instead, the endothermicity of the reaction is rate-limiting. Discussion about how energy, impact parameter, and angular momentum affect specific rate constants for dissociation to reactants, reaction to products, and association are presented.
中文翻译:

Co+(CH3Br)n (n = 0,1) 与溴甲烷反应性的实验和统计建模研究
Co + (CH 3 Br) n (n = 0,1) + CH 3 Br的温度和压力相关动力学在300 至 600 K 和 0.2 至 0.5 Torr 范围内测量。使用密度泛函计算解释结果并使用统计理论建模。在 n = 0 的情况下,缔合产物 Co + (CH 3 Br) 以碰撞值的 5% 到 30% 之间的速率常数占主导地位,而次要通道产生 CoBr + + CH 3,速率常数随温度增加。确定 Co + -Br (2.8 ± 0.1 eV) 和 Co + -CH 3 的0K 键解离能 (BDE)溴 (2.4 ± 0.25 eV)。Co + (即n = 1) 的连接显着增加了双分子反应速率常数。形成 CoBr + (CH 3 Br) + CH 3并且反应具有正温度依赖性。有趣的是,由于关联通道的竞争,该速率常数随着压力的增加而降低。连接增加了 (CH 3 Br)Co + -Br BDE (3.0 ± 0.1 eV) 相对于 Co + -Br,但降低了 (CH 3 Br)Co + -CH 3 Br BDE (1.95 ± 0.25) 相对于 Co + -CH 3兄弟 两种双分子反应都是通过金属插入机制进行的,具有不影响动力学的显着浸没过渡态。相反,反应的吸热性是限速的。讨论了能量、冲击参数和角动量如何影响分解为反应物、反应为产物和缔合的特定速率常数。










































 京公网安备 11010802027423号
京公网安备 11010802027423号