Chem Catalysis ( IF 11.5 ) Pub Date : 2021-05-10 , DOI: 10.1016/j.checat.2021.03.012 Dafeng Zhang , Junxiang Chen , Zhongjing Hao , Lei Jiao , Qingfeng Ge , Wen-Fu Fu , Xiao-Jun Lv
|
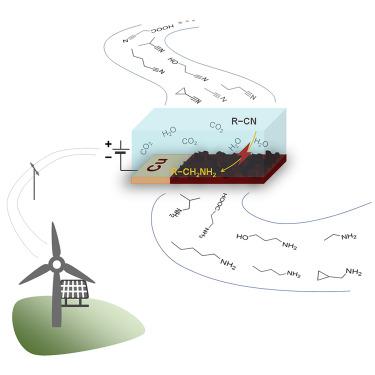
Selective nitrile hydrogenation to valuable primary amines under mild conditions is still a great challenge. Herein, we report the synergistic effect of a Cu catalyst and dissolved CO2 to electrochemically hydrogenate acetonitrile to ethylamine with a high selectivity (99%) and faradic efficiency (94%) in an aqueous electrolyte, superior to the best performance reported to date. The results show that a Cu catalyst offers preferential adsorption of the nitrile on the surface through the terminal C≡N group, facilitating the hydrogenation process on this site while suppressing the side reactions. The CO2 in the electrolyte protects the initially formed primary amine by reacting with the NH2 group, preventing it from condensing into amine dimers and trimers. Importantly, the method can be further extended to other aliphatic nitriles with functional groups, including carboxyl, hydroxyl, and cycloalkane, while exciting selectivity (>90%) of the corresponding primary amines was also obtained.
中文翻译:

乙腈高效电化学加氢制乙胺用于伯胺合成和储氢
在温和条件下选择性腈加氢生成有价值的伯胺仍然是一个巨大的挑战。在此,我们报告了 Cu 催化剂和溶解的 CO 2在水性电解质中以高选择性 (99%) 和法拉第效率 (94%) 电化学氢化乙腈为乙胺的协同效应,优于迄今为止报道的最佳性能。结果表明,Cu 催化剂通过末端 C≡N 基团优先吸附表面上的腈,促进该位点的氢化过程,同时抑制副反应。电解液中的 CO 2通过与 NH 2反应保护最初形成的伯胺基团,防止其缩合成胺二聚体和三聚体。重要的是,该方法可以进一步扩展到其他具有官能团的脂肪腈,包括羧基、羟基和环烷烃,同时还获得了相应伯胺的令人兴奋的选择性 (>90%)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号