当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Analysis of Solubility of 4-[2-(N-Methylcarbamyl)-4-pyridyloxy]aniline in Ten Pure Solvents and Three Binary Solvent Mixtures at Different Temperatures (T = 278.15–323.15 K)
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-14 , DOI: 10.1021/acs.jced.1c00232 Zhi Wang 1 , Liang Yao 1 , Ting Wang 1 , Zilong Cui 1 , Jingling Ye 1 , Yonghong Hu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-14 , DOI: 10.1021/acs.jced.1c00232 Zhi Wang 1 , Liang Yao 1 , Ting Wang 1 , Zilong Cui 1 , Jingling Ye 1 , Yonghong Hu 1
Affiliation
|
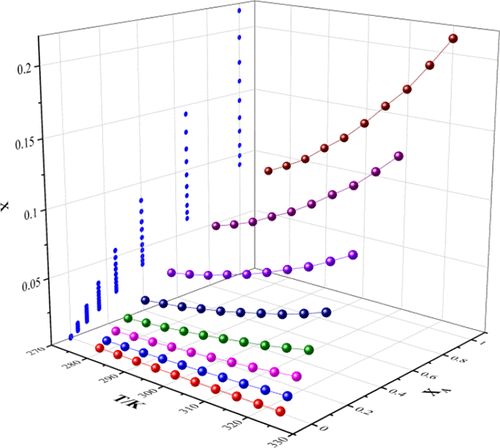
In this study, the data on solid–liquid equilibrium of 4-[2-(N-methylcarbamyl)-4-pyridyloxy]aniline were studied by the gravimetric method. The solubility of 4-[2-(N-methylcarbamyl)-4-pyridyloxy]aniline was measured in 10 pure solvents (ethyl acetate, tetrahydrofuran, acetonitrile, dichloromethane, acetone, methanol, acetylacetone, n-propanol, n-butanol, and water) and three binary solvent mixtures (water + tetrahydrofuran, acetone + n-butanol, and ethyl acetate + n-propanol) in the temperature range of 278.15 to 323.15 K under atmospheric pressure. The experimental results show that the solubility of 4-[2-(N-methylcarbamyl)-4-pyridyloxy]aniline in the abovementioned solvents increases with the increase of temperature. Among the 10 pure solvents, ethyl acetate has the highest solubility. The improved Apelblat model, Buchowski–Ksiazaczak λh model, Redlich–Kister (CNIBS/R-K) model, and Jouyban–Acree model are used for nonlinear fitting of experimental data, and the KAT-LSER model is also used to study the relationship between the solubility of 4-[2-(N-methylcarbamyl)-4-pyridyloxy]aniline in pure solvents and the interaction between solvent and solute molecules. The physical and chemical information of 4-[2-(N-methylcarbamyl)-4-pyridyloxy]aniline provided in this report may help its extraction/separation, recrystallization, purification, and formulation development.
中文翻译:

测定和分析 4-[2-(N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在十种纯溶剂和三种二元溶剂混合物中在不同温度 (T = 278.15–323.15 K) 中的溶解度
本研究采用重量法研究了4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺的固液平衡数据。4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在 10 种纯溶剂(乙酸乙酯、四氢呋喃、乙腈、二氯甲烷、丙酮、甲醇、乙酰丙酮、正丙醇、正丁醇和水)和三种二元溶剂混合物(水 + 四氢呋喃、丙酮 +正丁醇和乙酸乙酯 +正丙醇),温度范围为 278.15 至 323.15 K,常压。实验结果表明,4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在上述溶剂中随温度升高而增加。在 10 种纯溶剂中,乙酸乙酯的溶解度最高。改进的 Apelblat 模型、Buchowski-Ksiazaczak λ h模型、Redlich-Kister (CNIBS/RK) 模型和 Jouyban-Acree 模型用于实验数据的非线性拟合,KAT-LSER 模型也用于研究4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在纯溶剂中的溶解度以及溶剂与溶质分子之间的相互作用。本报告中提供的 4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺的物理和化学信息可能有助于其提取/分离、重结晶、纯化和制剂开发。
更新日期:2021-08-12
中文翻译:

测定和分析 4-[2-(N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在十种纯溶剂和三种二元溶剂混合物中在不同温度 (T = 278.15–323.15 K) 中的溶解度
本研究采用重量法研究了4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺的固液平衡数据。4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在 10 种纯溶剂(乙酸乙酯、四氢呋喃、乙腈、二氯甲烷、丙酮、甲醇、乙酰丙酮、正丙醇、正丁醇和水)和三种二元溶剂混合物(水 + 四氢呋喃、丙酮 +正丁醇和乙酸乙酯 +正丙醇),温度范围为 278.15 至 323.15 K,常压。实验结果表明,4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在上述溶剂中随温度升高而增加。在 10 种纯溶剂中,乙酸乙酯的溶解度最高。改进的 Apelblat 模型、Buchowski-Ksiazaczak λ h模型、Redlich-Kister (CNIBS/RK) 模型和 Jouyban-Acree 模型用于实验数据的非线性拟合,KAT-LSER 模型也用于研究4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺在纯溶剂中的溶解度以及溶剂与溶质分子之间的相互作用。本报告中提供的 4-[2-( N-甲基氨基甲酰基)-4-吡啶氧基]苯胺的物理和化学信息可能有助于其提取/分离、重结晶、纯化和制剂开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号