当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, insecticidal activities, and structure–activity relationships of 1,3,4-oxadiazole-ring-containing pyridylpyrazole-4-carboxamides as novel insecticides of the anthranilic diamide family
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-07-14 , DOI: 10.1002/jhet.4346 Abdalla Khallaf 1, 2 , Ping Wang 1 , Shuping Zhuo 1 , Hongjun Zhu 2 , Hui Liu 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-07-14 , DOI: 10.1002/jhet.4346 Abdalla Khallaf 1, 2 , Ping Wang 1 , Shuping Zhuo 1 , Hongjun Zhu 2 , Hui Liu 1
Affiliation
|
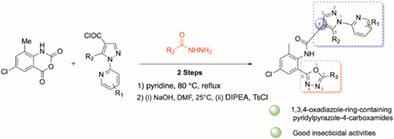
The preparation of novel anthranilic diamide derivatives is extremely important for agricultural pest control. In this study, pyridylpyrazole-4-carboxamides containing a 1,3,4-oxadiazole ring were designed and synthesized via the dehydration of aromatic hydrazine derivatives and formanilides in the presence of an alkali. The insecticidal activities of these new compounds against the diamondback moth (Plutella xylostella) were evaluated. N-(4-chloro-2-methyl-6-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)phenyl)-5-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carboxamide (8h6) showed 67%, 50%, 34%, 20%, and 17% activity at concentrations of 100, 50, 10, 5, and 1 μg ml−1, respectively. Density functional theory calculations showed that the introduction of the 1,3,4-oxadiazole ring significantly changed the electron distributions in both the highest occupied and lowest unoccupied molecular orbitals, resulting in these compounds having much larger energy gaps than the well-known insecticide chlorantraniliprole, which may account for their lower activity. The results of this study demonstrate that anthranilic diamides substituted with a 1,3,4-oxadiazole ring are effective insecticides that can be used for pest management.
中文翻译:

作为邻氨基苯甲酰胺家族新型杀虫剂的含 1,3,4-恶二唑环的吡啶基吡唑-4-甲酰胺的合成、杀虫活性和构效关系
新型邻氨基苯甲酰胺衍生物的制备对于农业害虫防治极为重要。在这项研究中,通过芳香肼衍生物和甲酰苯胺在碱存在下的脱水,设计并合成了含有 1,3,4-恶二唑环的吡啶基吡唑-4-甲酰胺。评估了这些新化合物对小菜蛾(Plutella xylostella)的杀虫活性。N-(4-chloro-2-methyl-6-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)phenyl)-5-methyl-1-(pyridin-2-yl) -1H-吡唑-4-甲酰胺 ( 8h6 ) 在100、50、10、5和 1 μg ml -1 的浓度下显示出 67%、50%、34%、20% 和 17% 的活性, 分别。密度泛函理论计算表明,1,3,4-恶二唑环的引入显着改变了最高占据和最低未占据分子轨道的电子分布,导致这些化合物的能隙比众所周知的杀虫剂氯虫苯甲酰胺大得多,这可能是它们较低的活性的原因。本研究的结果表明,被 1,3,4-恶二唑环取代的邻氨基苯甲酰胺是可用于害虫管理的有效杀虫剂。
更新日期:2021-07-14
中文翻译:

作为邻氨基苯甲酰胺家族新型杀虫剂的含 1,3,4-恶二唑环的吡啶基吡唑-4-甲酰胺的合成、杀虫活性和构效关系
新型邻氨基苯甲酰胺衍生物的制备对于农业害虫防治极为重要。在这项研究中,通过芳香肼衍生物和甲酰苯胺在碱存在下的脱水,设计并合成了含有 1,3,4-恶二唑环的吡啶基吡唑-4-甲酰胺。评估了这些新化合物对小菜蛾(Plutella xylostella)的杀虫活性。N-(4-chloro-2-methyl-6-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)phenyl)-5-methyl-1-(pyridin-2-yl) -1H-吡唑-4-甲酰胺 ( 8h6 ) 在100、50、10、5和 1 μg ml -1 的浓度下显示出 67%、50%、34%、20% 和 17% 的活性, 分别。密度泛函理论计算表明,1,3,4-恶二唑环的引入显着改变了最高占据和最低未占据分子轨道的电子分布,导致这些化合物的能隙比众所周知的杀虫剂氯虫苯甲酰胺大得多,这可能是它们较低的活性的原因。本研究的结果表明,被 1,3,4-恶二唑环取代的邻氨基苯甲酰胺是可用于害虫管理的有效杀虫剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号