当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disruption of Metallostasis in the Anaerobic Human Pathogen Fusobacterium nucleatum by the Zinc Ionophore PBT2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-07-14 , DOI: 10.1021/acsinfecdis.0c00887 Essie M Van Zuylen 1 , Scott A Ferguson 1 , Alan Hughes 1 , David Rennison 2 , Margaret A Brimble 2, 3 , Gregory M Cook 1, 3
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-07-14 , DOI: 10.1021/acsinfecdis.0c00887 Essie M Van Zuylen 1 , Scott A Ferguson 1 , Alan Hughes 1 , David Rennison 2 , Margaret A Brimble 2, 3 , Gregory M Cook 1, 3
Affiliation
|
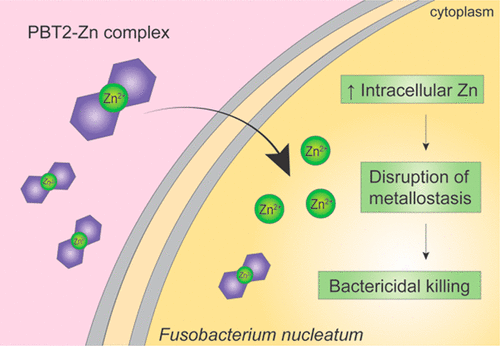
The Gram-negative anaerobe Fusobacterium nucleatum is an opportunistic human pathogen, most frequently associated with periodontal disease through dental biofilm formation and, increasingly, with colorectal cancer development and progression. F. nucleatum infections are routinely treated by broad-spectrum β-lactam antibiotics and metronidazole. However, these antibiotics can negatively impact the normal microflora. Therefore, the development of novel narrow-spectrum antimicrobials active against anaerobic pathogens is of great interest. Here, we examined the antimicrobial Zn ionophore PBT2, an 8-hydroxyquinoline analogue with metal chelating properties, against a single type isolate F. nucleatum ATCC 25586. PBT2-Zn was a potent inhibitor of growth and exhibited synergistic bactericidal (>3-log10 killing) activity at 5× MIC in planktonic cells, and at the MIC in biofilms grown in vitro. Physiological and transcriptional analyses uncovered a strong cellular response relating to Zn and Fe homeostasis in PBT2-Zn treated cells across subinhibitory and inhibitory concentrations. At 1× MIC, PBT2 alone induced a 3.75-fold increase in intracellular Zn, whereas PBT2-Zn challenge induced a 19-fold accumulation of intracellular Zn after 2 h. A corresponding 2.1-fold loss of Fe was observed at 1× MIC. Transcriptional analyses after subinhibitory PBT2-Zn challenge (0.125 μg/mL and 200 μM ZnSO4) revealed significant differential expression of 15 genes at 0.5 h, and 12 genes at 1 h. Upregulated genes included those with roles in Zn homeostasis (e.g., a Zn-transporting ATPase and the Zn-sensing transcriptional regulator, smtB) and hemin transport (hmuTUV) to re-establish Fe homeostasis. A concentration-dependent protective effect was observed for cells pretreated with hemin (50 μg/mL) prior to PBT2-Zn challenge. The data presented here supports our proposal that targeting the disruption of metallostasis by Zn-translocating ionophores is a strategy worth investigating further for the treatment of Gram-negative anaerobic pathogens.
中文翻译:

锌离子载体 PBT2 破坏人类厌氧病原体具核梭杆菌中的金属稳态
革兰氏阴性厌氧菌具核梭杆菌是一种机会性人类病原体,最常通过牙齿生物膜形成与牙周病相关,并且越来越多地与结直肠癌的发展和进展相关。F. nucleatum感染通常用广谱 β-内酰胺抗生素和甲硝唑治疗。然而,这些抗生素会对正常微生物群产生负面影响。因此,开发对厌氧病原体有活性的新型窄谱抗菌剂具有重要意义。在这里,我们检查了抗微生物锌离子载体 PBT2,一种具有金属螯合特性的 8-羟基喹啉类似物,针对单一类型的分离物F. nucleatumATCC 25586。PBT2-Zn 是一种有效的生长抑制剂,在浮游细胞 5 倍 MIC 和体外生长的生物膜 MIC 下表现出协同杀菌(>3-log 10杀灭)活性。生理学和转录分析揭示了在 PBT2-Zn 处理的细胞中,在亚抑制和抑制浓度下,与 Zn 和 Fe 稳态相关的强烈细胞反应。在 1 倍 MIC 下,PBT2 单独诱导细胞内锌增加 3.75 倍,而 PBT2-Zn 激发在 2 小时后诱导细胞内锌积累 19 倍。在 1×MIC 下观察到相应的 Fe 损失 2.1 倍。亚抑制性 PBT2-Zn 激发后的转录分析(0.125 μg/mL 和 200 μM ZnSO 4) 在 0.5 小时显示 15 个基因的显着差异表达,在 1 小时显示 12 个基因的显着差异表达。上调的基因包括在锌稳态中发挥作用的基因(例如,锌转运 ATP 酶和锌感应转录调节因子smtB)和血红素转运 ( hmuTUV ) 以重新建立铁稳态。在 PBT2-Zn 攻击之前用氯化血红素 (50 μg/mL) 预处理的细胞观察到浓度依赖性保护作用。此处提供的数据支持我们的提议,即通过 Zn 易位离子载体破坏金属稳态是一种值得进一步研究用于治疗革兰氏阴性厌氧病原体的策略。
更新日期:2021-08-13
中文翻译:

锌离子载体 PBT2 破坏人类厌氧病原体具核梭杆菌中的金属稳态
革兰氏阴性厌氧菌具核梭杆菌是一种机会性人类病原体,最常通过牙齿生物膜形成与牙周病相关,并且越来越多地与结直肠癌的发展和进展相关。F. nucleatum感染通常用广谱 β-内酰胺抗生素和甲硝唑治疗。然而,这些抗生素会对正常微生物群产生负面影响。因此,开发对厌氧病原体有活性的新型窄谱抗菌剂具有重要意义。在这里,我们检查了抗微生物锌离子载体 PBT2,一种具有金属螯合特性的 8-羟基喹啉类似物,针对单一类型的分离物F. nucleatumATCC 25586。PBT2-Zn 是一种有效的生长抑制剂,在浮游细胞 5 倍 MIC 和体外生长的生物膜 MIC 下表现出协同杀菌(>3-log 10杀灭)活性。生理学和转录分析揭示了在 PBT2-Zn 处理的细胞中,在亚抑制和抑制浓度下,与 Zn 和 Fe 稳态相关的强烈细胞反应。在 1 倍 MIC 下,PBT2 单独诱导细胞内锌增加 3.75 倍,而 PBT2-Zn 激发在 2 小时后诱导细胞内锌积累 19 倍。在 1×MIC 下观察到相应的 Fe 损失 2.1 倍。亚抑制性 PBT2-Zn 激发后的转录分析(0.125 μg/mL 和 200 μM ZnSO 4) 在 0.5 小时显示 15 个基因的显着差异表达,在 1 小时显示 12 个基因的显着差异表达。上调的基因包括在锌稳态中发挥作用的基因(例如,锌转运 ATP 酶和锌感应转录调节因子smtB)和血红素转运 ( hmuTUV ) 以重新建立铁稳态。在 PBT2-Zn 攻击之前用氯化血红素 (50 μg/mL) 预处理的细胞观察到浓度依赖性保护作用。此处提供的数据支持我们的提议,即通过 Zn 易位离子载体破坏金属稳态是一种值得进一步研究用于治疗革兰氏阴性厌氧病原体的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号