Structure ( IF 4.4 ) Pub Date : 2021-07-14 , DOI: 10.1016/j.str.2021.06.014 Domenica Farci 1 , Sami Kereïche 2 , Sushil Pangeni 3 , Patrycja Haniewicz 1 , Igor V Bodrenko 4 , Matteo Ceccarelli 4 , Mathias Winterhalter 3 , Dario Piano 5
|
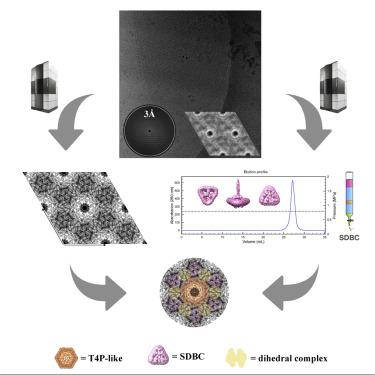
Bacterial surface layers are paracrystalline assemblies of proteins that provide the first line of defense against environmental shocks. Here, we report the 3D structure, in situ localization, and orientation of the S-layer deinoxanthin-binding complex (SDBC), a hetero-oligomeric assembly of proteins that in Deinococcus radiodurans represents the main S-layer unit. The SDBC is resolved at 11-Å resolution by single-particle analysis, while its in situ localization is determined by cryo-electron crystallography on intact cell-wall fragments leading to a projection map at 4.5-Å resolution. The SDBC exhibits a triangular base with three comma-shaped pores, and a stalk departing orthogonally from the center of the base and oriented toward the intracellular space. Combining state-of-the-art techniques, results show the organization of this S-layer and its connection within the underlying membranes, demonstrating the potential for applications from nanotechnologies to medicine.
中文翻译:

耐辐射球菌主要 S 层复合物结构和原位定位的结构分析
细菌表面层是蛋白质的准晶体组装体,可提供抵御环境冲击的第一道防线。在这里,我们报告了 S 层抗黄质结合复合物 (SDBC)的 3D 结构、原位定位和方向,SDBC 是一种异质寡聚蛋白组装体,在抗辐射球菌中代表主要的 S 层单元。SDBC 通过单粒子分析以 11 Å 分辨率解析,而其原位定位是由完整细胞壁碎片上的低温电子晶体学确定的,从而产生 4.5-Å 分辨率的投影图。SDBC 呈三角形基部,具有三个逗号形孔,茎部从基部中心正交偏离并朝向细胞内空间。结合最先进的技术,结果显示了这个 S 层的组织及其在下层膜内的连接,展示了从纳米技术到医学的应用潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号