当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Data Correlation of Metformin Hydrochloride in Four Aqueous Binary Solvents and Three Pure Solvents from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-07-13 , DOI: 10.1021/acs.jced.1c00338 Xinyuan Sun 1 , Shichao Du 1 , Ying Sun 2 , Hongcheng Li 3 , ChuanPing Yu 1 , Jianhui Guo 3 , Yan Wang 1 , Shuai Yu 1 , Yan Cheng 1 , Fumin Xue 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-07-13 , DOI: 10.1021/acs.jced.1c00338 Xinyuan Sun 1 , Shichao Du 1 , Ying Sun 2 , Hongcheng Li 3 , ChuanPing Yu 1 , Jianhui Guo 3 , Yan Wang 1 , Shuai Yu 1 , Yan Cheng 1 , Fumin Xue 1
Affiliation
|
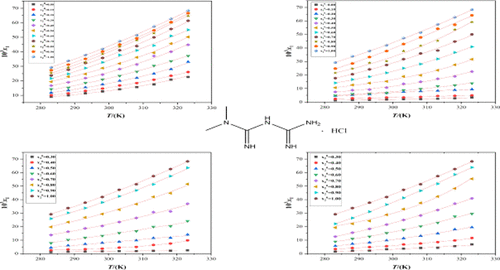
The solubility of form A metformin hydrochloride (MET·HCl) in water, methanol, ethanol, water + methanol, water + ethanol, water + acetone, and water + isopropanol at the temperatures ranging from 283.15 to 323.15 K was determined by gravimetric method. The experimental results show that the solubility increases with the increase of temperature and the initial water content of the binary solvents. The order of solubility in the four aqueous solvent mixtures is water + methanol > water + ethanol > water + isopropanol > water + acetone, which is mainly contributed by the hydrogen bond interactions. The modified Apelblat model, CNIBS/R–K model, and Apelblat–Jouyban–Acree model were used to correlate the solubility data. The results show that the Apelblat model can correlate the solubility of four binary solvents best, and all average relative deviations are less than 6%.
中文翻译:

盐酸二甲双胍在 283.15 至 323.15 K 的四种水性二元溶剂和三种纯溶剂中的溶解度测量和数据相关性
A型二甲双胍盐酸盐(MET·HCl)在水、甲醇、乙醇、水+甲醇、水+乙醇、水+丙酮和水+异丙醇中在283.15至323.15 K的温度范围内的溶解度通过重量法测定。实验结果表明,溶解度随着温度的升高和二元溶剂初始含水量的增加而增加。在四种水性溶剂混合物中的溶解度顺序为水+甲醇>水+乙醇>水+异丙醇>水+丙酮,主要由氢键相互作用贡献。修正的 Apelblat 模型、CNIBS/R-K 模型和 Apelblat-Jouyban-Acree 模型用于关联溶解度数据。结果表明,Apelblat 模型可以最好地关联四种二元溶剂的溶解度,
更新日期:2021-08-12
中文翻译:

盐酸二甲双胍在 283.15 至 323.15 K 的四种水性二元溶剂和三种纯溶剂中的溶解度测量和数据相关性
A型二甲双胍盐酸盐(MET·HCl)在水、甲醇、乙醇、水+甲醇、水+乙醇、水+丙酮和水+异丙醇中在283.15至323.15 K的温度范围内的溶解度通过重量法测定。实验结果表明,溶解度随着温度的升高和二元溶剂初始含水量的增加而增加。在四种水性溶剂混合物中的溶解度顺序为水+甲醇>水+乙醇>水+异丙醇>水+丙酮,主要由氢键相互作用贡献。修正的 Apelblat 模型、CNIBS/R-K 模型和 Apelblat-Jouyban-Acree 模型用于关联溶解度数据。结果表明,Apelblat 模型可以最好地关联四种二元溶剂的溶解度,



























 京公网安备 11010802027423号
京公网安备 11010802027423号