当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of atrazine in the electrochemical LED-UV/Cl2 system: the role of ˙OH and Cl˙
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2021-06-28 , DOI: 10.1039/d1ew00039j Ying Huang 1 , Yangyang Li 1 , Minghao Kong 2 , Dionysios D. Dionysiou 2 , Lecheng Lei 1, 3
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2021-06-28 , DOI: 10.1039/d1ew00039j Ying Huang 1 , Yangyang Li 1 , Minghao Kong 2 , Dionysios D. Dionysiou 2 , Lecheng Lei 1, 3
Affiliation
|
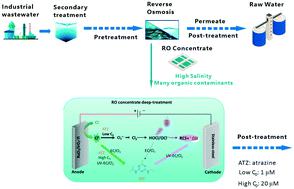
In this study, electrochemically generated free chlorine (EC/Cl2) was activated by UV275nm irradiation to degrade atrazine, a commonly used herbicide, investigating the degradation kinetics and mechanisms. The potential at a RuO2/IrO2–Ti anode was optimized as 1.5 V vs. Ag/AgCl through electrochemical measurement methods. The effects of atrazine initial concentration (C0) on the UV-EC/Cl2 and EC/Cl2 processes were evaluated. The atrazine degradation efficiency under low C0 conditions is surprisingly distinct from that under high C0 conditions. Under low C0 (1 μM) conditions, UV-EC/Cl2 degraded 60% atrazine at 60 min with ˙OH as the dominant species, while EC/Cl2 only removed 6% atrazine within 60 min. Under high C0 (20 μM) conditions, similar atrazine degradation efficiencies (about 90% within 180 min) were observed both in UV-EC/Cl2 and EC/Cl2. 50 μM nitrobenzene was used with 20 μM atrazine to conduct competition experiments, which confirmed the significant role of Cl˙ in atrazine degradation in the UV-EC/Cl2 and EC/Cl2 systems. During atrazine degradation by UV-EC/Cl2 and EC/Cl2, transformation products were compared according to high-resolution mass spectra, based on which possible degradation pathways were proposed. Synthetic reverse osmosis concentrate (ROC) was used to mimic the ROC from industrial wastewater and roughly evaluate the performances of UV-EC/Cl2 for the degradation of emerging contaminants, including atrazine, ibuprofen, carbamazepine, and diclofenac. Overall, EC/Cl2 can be effective at optimized anodic potential for the degradation of contaminants that are at high C0 and quickly react with Cl˙, while UV-EC/Cl2 is able to generate ˙OH and reactive chlorine species to efficiently decompose organic contaminants at varied concentrations.
中文翻译:

电化学 LED-UV/Cl2 系统中莠去津的降解:˙OH 和 Cl˙ 的作用
在这项研究中,电化学产生的游离氯 (EC/Cl 2 ) 被紫外线275nm照射激活以降解莠去津,一种常用的除草剂,研究降解动力学和机制。通过电化学测量方法,RuO 2 /IrO 2 -Ti 阳极的电位优化为 1.5 V vs. Ag/AgCl。评估了阿特拉津初始浓度 ( C 0 ) 对 UV-EC/Cl 2和 EC/Cl 2过程的影响。低下阿特拉津降解效率Ç 0的条件是从下高令人惊奇地不同Ç 0使适应。在低C 0 (1 μM) 条件下,UV-EC/Cl 2在 60 分钟时降解了 60% 的莠去津,其中 ˙OH 作为主要物质,而 EC/Cl 2在 60 分钟内仅去除了 6% 的莠去津。在高C 0 (20 μM) 条件下,在 UV-EC/Cl 2和 EC/Cl 2中观察到类似的莠去津降解效率(180 分钟内约 90%)。50 μM硝基苯与20 μM莠去津进行竞争实验,证实了Cl·在UV-EC/Cl 2和EC/Cl 2系统中阿特拉津降解中的重要作用。在 UV-EC/Cl 2和 EC/Cl 2降解莠去津期间,根据高分辨率质谱比较了转化产物,在此基础上提出了可能的降解途径。合成反渗透浓缩物 (ROC) 用于模拟工业废水中的 ROC,并粗略评估 UV-EC/Cl 2降解新兴污染物的性能,包括阿特拉津、布洛芬、卡马西平和双氯芬酸。总体而言,EC/Cl 2可以有效地优化阳极电位,用于降解高C 0并与 Cl 快速反应的污染物,而 UV-EC/Cl 2能够生成 ˙OH 和活性氯物质以有效地分解不同浓度的有机污染物。
更新日期:2021-07-13
中文翻译:

电化学 LED-UV/Cl2 系统中莠去津的降解:˙OH 和 Cl˙ 的作用
在这项研究中,电化学产生的游离氯 (EC/Cl 2 ) 被紫外线275nm照射激活以降解莠去津,一种常用的除草剂,研究降解动力学和机制。通过电化学测量方法,RuO 2 /IrO 2 -Ti 阳极的电位优化为 1.5 V vs. Ag/AgCl。评估了阿特拉津初始浓度 ( C 0 ) 对 UV-EC/Cl 2和 EC/Cl 2过程的影响。低下阿特拉津降解效率Ç 0的条件是从下高令人惊奇地不同Ç 0使适应。在低C 0 (1 μM) 条件下,UV-EC/Cl 2在 60 分钟时降解了 60% 的莠去津,其中 ˙OH 作为主要物质,而 EC/Cl 2在 60 分钟内仅去除了 6% 的莠去津。在高C 0 (20 μM) 条件下,在 UV-EC/Cl 2和 EC/Cl 2中观察到类似的莠去津降解效率(180 分钟内约 90%)。50 μM硝基苯与20 μM莠去津进行竞争实验,证实了Cl·在UV-EC/Cl 2和EC/Cl 2系统中阿特拉津降解中的重要作用。在 UV-EC/Cl 2和 EC/Cl 2降解莠去津期间,根据高分辨率质谱比较了转化产物,在此基础上提出了可能的降解途径。合成反渗透浓缩物 (ROC) 用于模拟工业废水中的 ROC,并粗略评估 UV-EC/Cl 2降解新兴污染物的性能,包括阿特拉津、布洛芬、卡马西平和双氯芬酸。总体而言,EC/Cl 2可以有效地优化阳极电位,用于降解高C 0并与 Cl 快速反应的污染物,而 UV-EC/Cl 2能够生成 ˙OH 和活性氯物质以有效地分解不同浓度的有机污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号