当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orthogonal Enzymatic Conjugation Reactions Create Chitin Binding Domain Grafted Chitinase Polymers with Enhanced Antifungal Activity
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-07-12 , DOI: 10.1021/acs.bioconjchem.1c00235 Kosuke Minamihata 1 , Yusuke Tanaka 1 , Pugoh Santoso 1 , Masahiro Goto 1, 2 , Dan Kozome 3 , Toki Taira 3 , Noriho Kamiya 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-07-12 , DOI: 10.1021/acs.bioconjchem.1c00235 Kosuke Minamihata 1 , Yusuke Tanaka 1 , Pugoh Santoso 1 , Masahiro Goto 1, 2 , Dan Kozome 3 , Toki Taira 3 , Noriho Kamiya 1, 2
Affiliation
|
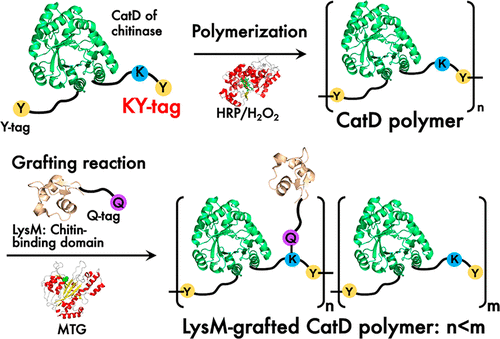
Enzymatic reaction offers site-specific conjugation of protein units to form protein conjugates or protein polymers with intrinsic functions. Herein, we report horseradish peroxidase (HRP)- and microbial transglutaminase (MTG)-catalyzed orthogonal conjugation reactions to create antifungal protein polymers composed of Pteris ryukyuensis chitinase-A (ChiA) and its two domains, catalytic domain, CatD, and chitin-binding domain, LysM2. We engineered the ChiA and CatD by introducing a peptide tag containing tyrosine (Y-tag) at N-termini and a peptide tag containing lysine and tyrosine (KY-tag) at C-termini to construct Y-ChiA-KY and Y-CatD-KY. Also, LysM2 with Y-tag and KY-tag (Y-LysM2-KY) or with a glutamine-containing peptide tag (Q-tag) (LysM2-Q) were constructed. The proteins with Y-tag and KY-tag were efficiently polymerized by HRP reaction through the formation of dityrosine bonds at the tyrosine residues in the peptide tags. The Y-CatD-KY polymer was further treated by MTG to orthogonally graft LysM2-Q to the KY-tag via isopeptide formation between the side chains of the glutamine and lysine residues in the peptide tags to form LysM2-grafted CatD polymer. The LysM2-grafted CatD polymer exhibited significantly higher antifungal activity than the homopolymer of Y-ChiA-KY and the random copolymer of Y-CatD-KY and Y-LysM2-KY, demonstrating that the structural differences of artificial chitinase polymers have a significant impact on the antifungal activity. This strategy of polymerization and grafting reaction of protein can contribute to the further research and development of functional protein polymers for specific applications in various fields in biotechnology.
中文翻译:

正交酶促偶联反应产生具有增强抗真菌活性的几丁质结合域接枝几丁质酶聚合物
酶促反应提供蛋白质单位的位点特异性结合,以形成具有内在功能的蛋白质结合物或蛋白质聚合物。在本文中,我们报道了辣根过氧化物酶(HRP) -和微生物转谷氨酰胺酶(MTG)催化正交缀合反应以产生组成的抗真菌蛋白质聚合物凤尾ryukyuensis几丁质酶A(嘉)和它的两个结构域,催化结构域,CATD,和几丁质结合域,LysM 2。我们通过在 N 端引入含有酪氨酸 (Y-tag) 的肽标签和在 C 端引入含有赖氨酸和酪氨酸的肽标签 (KY-tag) 来设计 ChiA 和 CatD,以构建 Y-ChiA-KY 和 Y-CatD -KY。此外,LysM 2带有 Y 标签和 KY 标签(Y-LysM 2-KY) 或含有谷氨酰胺的肽标签 (Q-tag) (LysM 2 -Q) 被构建。带有 Y 标签和 KY 标签的蛋白质通过 HRP 反应通过在肽标签中的酪氨酸残基处形成二酪氨酸键而有效聚合。Y-CatD-KY 聚合物通过 MTG 进一步处理,通过在谷氨酰胺侧链和肽标签中的赖氨酸残基之间形成异肽,将LysM 2 -Q正交接枝到 KY 标签,以形成 LysM 2接枝的 CatD 聚合物。LysM 2接枝的 CatD 聚合物表现出比 Y-ChiA-KY 的均聚物和 Y-CatD-KY 和 Y-LysM 2的无规共聚物显着更高的抗真菌活性-KY,表明人工几丁质酶聚合物的结构差异对抗真菌活性有显着影响。这种蛋白质聚合和接枝反应的策略有助于进一步研究和开发用于生物技术各个领域特定应用的功能性蛋白质聚合物。
更新日期:2021-08-19
中文翻译:

正交酶促偶联反应产生具有增强抗真菌活性的几丁质结合域接枝几丁质酶聚合物
酶促反应提供蛋白质单位的位点特异性结合,以形成具有内在功能的蛋白质结合物或蛋白质聚合物。在本文中,我们报道了辣根过氧化物酶(HRP) -和微生物转谷氨酰胺酶(MTG)催化正交缀合反应以产生组成的抗真菌蛋白质聚合物凤尾ryukyuensis几丁质酶A(嘉)和它的两个结构域,催化结构域,CATD,和几丁质结合域,LysM 2。我们通过在 N 端引入含有酪氨酸 (Y-tag) 的肽标签和在 C 端引入含有赖氨酸和酪氨酸的肽标签 (KY-tag) 来设计 ChiA 和 CatD,以构建 Y-ChiA-KY 和 Y-CatD -KY。此外,LysM 2带有 Y 标签和 KY 标签(Y-LysM 2-KY) 或含有谷氨酰胺的肽标签 (Q-tag) (LysM 2 -Q) 被构建。带有 Y 标签和 KY 标签的蛋白质通过 HRP 反应通过在肽标签中的酪氨酸残基处形成二酪氨酸键而有效聚合。Y-CatD-KY 聚合物通过 MTG 进一步处理,通过在谷氨酰胺侧链和肽标签中的赖氨酸残基之间形成异肽,将LysM 2 -Q正交接枝到 KY 标签,以形成 LysM 2接枝的 CatD 聚合物。LysM 2接枝的 CatD 聚合物表现出比 Y-ChiA-KY 的均聚物和 Y-CatD-KY 和 Y-LysM 2的无规共聚物显着更高的抗真菌活性-KY,表明人工几丁质酶聚合物的结构差异对抗真菌活性有显着影响。这种蛋白质聚合和接枝反应的策略有助于进一步研究和开发用于生物技术各个领域特定应用的功能性蛋白质聚合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号