Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-07-13 , DOI: 10.1016/j.jmgm.2021.107984 Xinrong Zhuang 1 , Xuefeng Shen 1 , Wensi Niu 1 , Lingjun Kong 1
|
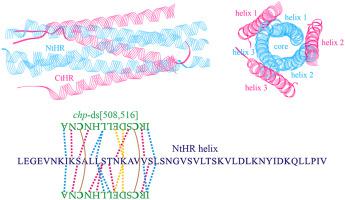
Human respiratory syncytial virus (RSV) is the major cause of acute lower respiratory tract infections worldwide in infants and young children. The RSV F glycoprotein is a class I fusion protein that mediates viral entry into host cells and is a major target of neutralizing antibodies. Targeting F glycoprotein has been recognized as a promising antiviral therapeutic strategy against RSV infection. Here, we reported the disulfide-stapled design of α-helical bundle to target the trimer-of-hairpins (TOH) motif of RSV F glycoprotein, which is the central regulatory module that triggers viral membrane fusion event. In TOH motif, three N-terminal heptad repeat (NtHR) helices form a trimeric coiled-coil core and other three C-terminal heptad repeat (CtHR) helices add to the core in an antiparallel manner. Interaction analysis between NtHR and CtHR revealed that the C-terminal tail of CtHR packs tightly against NtHR as compared to the N-terminal and middle regions of CtHR. A core binding site in CtHR C-terminus was identified, which represents a 13-mer chp peptide and can effectively interact with NtHR helix in native ordered conformation but would become largely disordered when splitting from the protein context of CtHR helix. Two chp helices were stapled together in a parallel manner with single, double or triple disulfide bridges, thus systematically resulting in seven disulfide-stapled α-helical bundles. Molecular simulations revealed that the double and triple stapling can effectively stabilize the structured conformation of α-helical bundles, whereas the free conformation of single-stapled bundles still remain intrinsically disordered in solvent. The double-stapled bundle chp-ds[508,516] and the triple-stapled bundle chp-ts[508,512,516] were rationally designed to have high potency; they can form a tight three-helix bundle with NtHR helix, thus potently targeting NtHR–CtHR interactions involved in RSV-F TOH motif through a competitive disruption mechanism.
中文翻译:

靶向人呼吸道合胞病毒融合蛋白三聚体发夹基序的α-螺旋束的二硫化物钉合设计
人类呼吸道合胞病毒 (RSV) 是全球婴幼儿急性下呼吸道感染的主要原因。RSV F 糖蛋白是一种 I 类融合蛋白,可介导病毒进入宿主细胞,是中和抗体的主要目标。靶向 F 糖蛋白已被认为是一种有前途的抗 RSV 感染的抗病毒治疗策略。在这里,我们报告了 α-螺旋束的二硫化物钉合设计,以靶向 RSV F 糖蛋白的发夹三聚体 (TOH) 基序,这是触发病毒膜融合事件的中央调节模块。在 TOH 基序中,三个 N 端七肽重复 (NtHR) 螺旋形成一个三聚体卷曲螺旋核心,其他三个 C 端七肽重复 (CtHR) 螺旋以反平行方式添加到核心中。NtHR 和 CtHR 之间的相互作用分析表明,与 CtHR 的 N 末端和中间区域相比,CtHR 的 C 末端尾部与 NtHR 紧密结合。鉴定了 CtHR C 端的核心结合位点,代表 13 聚体chp肽并且可以有效地与天然有序构象中的 NtHR 螺旋相互作用,但当从 CtHR 螺旋的蛋白质背景中分裂出来时,会变得很无序。两个chp螺旋以平行的方式用单、双或三重二硫键连接在一起,从而系统地产生七个二硫键连接的 α-螺旋束。分子模拟表明,双钉和三钉可以有效地稳定α-螺旋束的结构构象,而单钉束的自由构象在溶剂中仍然保持本质上的无序。双订书包chp -ds[508,516] 和三订书包chp-ts[508,512,516] 被合理设计为具有高效力;它们可以与 NtHR 螺旋形成紧密的三螺旋束,从而通过竞争性破坏机制有效地靶向 RSV-F TOH 基序中涉及的 NtHR-CtHR 相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号