当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Six-Step Gram-Scale Synthesis of the Human Immunodeficiency Virus Integrase Inhibitor Dolutegravir Sodium
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-07-12 , DOI: 10.1021/acs.oprd.1c00139 Jule-Philipp Dietz 1 , Tobias Lucas 1 , Jonathan Groß 1 , Sebastian Seitel 1 , Jan Brauer 1 , Dorota Ferenc 1 , B. Frank Gupton 2 , Till Opatz 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-07-12 , DOI: 10.1021/acs.oprd.1c00139 Jule-Philipp Dietz 1 , Tobias Lucas 1 , Jonathan Groß 1 , Sebastian Seitel 1 , Jan Brauer 1 , Dorota Ferenc 1 , B. Frank Gupton 2 , Till Opatz 1
Affiliation
|
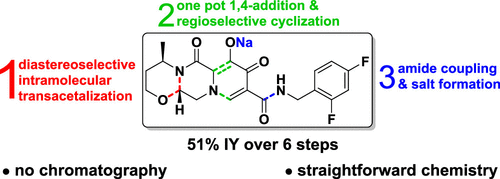
A short and practical synthesis for preparing the active pharmaceutical ingredient dolutegravir sodium was developed. The convergent strategy starts from (R)-3-amino-1-butanol and establishes the BC ring system in a 76% isolated yield over four steps. Ring A was constructed by a one-pot 1,4-addition to diethyl-(2E/Z)-2-(ethoxymethylidene)-3-oxobutandioate and subsequent MgBr2·OEt2-mediated regioselective cyclization. Amide formation with 2,4-difluorobenzylamine was either performed from the free carboxylic acid or through aminolysis of the corresponding ethyl ester. Final salt formation afforded dolutegravir sodium in a 48–51% isolated yield (HPLC purity of 99.7–99.9%) over six linear steps.
中文翻译:

人类免疫缺陷病毒整合酶抑制剂多替拉韦钠的六步革兰氏规模合成
开发了一种用于制备活性药物成分多替拉韦钠的简短实用的合成方法。收敛策略从 ( R )-3-氨基-1-丁醇开始,并在四个步骤中以 76% 的分离产率建立了 BC 环系统。通过一锅1,4-加成到二乙基-( 2E / Z )-2-(乙氧基亚甲基)-3-氧代丁二酸和随后的MgBr 2 ·OEt 2介导的区域选择性环化来构建环A。与 2,4-二氟苄胺形成酰胺是通过游离羧酸或通过相应乙酯的氨解进行的。最终的盐形成通过六个线性步骤以 48-51% 的分离产率(HPLC 纯度为 99.7-99.9%)提供了多替拉韦钠。
更新日期:2021-08-20
中文翻译:

人类免疫缺陷病毒整合酶抑制剂多替拉韦钠的六步革兰氏规模合成
开发了一种用于制备活性药物成分多替拉韦钠的简短实用的合成方法。收敛策略从 ( R )-3-氨基-1-丁醇开始,并在四个步骤中以 76% 的分离产率建立了 BC 环系统。通过一锅1,4-加成到二乙基-( 2E / Z )-2-(乙氧基亚甲基)-3-氧代丁二酸和随后的MgBr 2 ·OEt 2介导的区域选择性环化来构建环A。与 2,4-二氟苄胺形成酰胺是通过游离羧酸或通过相应乙酯的氨解进行的。最终的盐形成通过六个线性步骤以 48-51% 的分离产率(HPLC 纯度为 99.7-99.9%)提供了多替拉韦钠。



























 京公网安备 11010802027423号
京公网安备 11010802027423号