当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the Origin of E-Selectivity in the Ring-Opening Metathesis Polymerization with Molybdenum Imido Alkylidene N-Heterocyclic Carbene Complexes
Organometallics ( IF 2.8 ) Pub Date : 2021-07-09 , DOI: 10.1021/acs.organomet.1c00229 Maren Podewitz 1 , Suman Sen 2 , Michael R Buchmeiser 2
Organometallics ( IF 2.8 ) Pub Date : 2021-07-09 , DOI: 10.1021/acs.organomet.1c00229 Maren Podewitz 1 , Suman Sen 2 , Michael R Buchmeiser 2
Affiliation
|
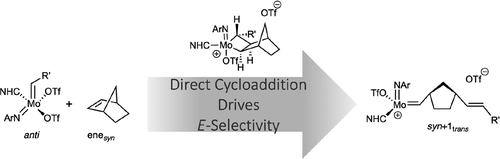
The understanding and control of stereoselectivity is a central aspect in ring-opening metathesis polymerization (ROMP). Herein, we report detailed quantum chemical studies on the reaction mechanism of E-selective ROMP of norborn-2-ene (NBE) with Mo(N-2,6-Me2-C6H3)(CHCMe3)(IMes)(OTf)2 (1, IMes = 1,3-dimesitylimidazol-2-ylidene) as a first step to stereoselective polymerization. Four different reaction pathways based on an enesyn or eneanti approach of NBE to either the syn- or anti-isomer of the neutral precatalyst have been studied. In contrast to the recently established associative mechanism with a terminal alkene, where a neutral olefin adduct is formed, NBE reacts directly with the catalyst via [2 + 2] cycloaddition to form molybdacyclobutane with a reaction barrier about 30 kJ mol–1 lower in free energy than via the formation of a catalyst–monomer adduct. However, the direct cycloaddition of NBE was only found for one out of four stereoisomers. Our findings strongly suggest that this stereoselective approach is responsible for E-selectivity and point toward a substrate-specific reaction mechanism in olefin metathesis with neutral Mo imido alkylidene N-heterocyclic carbene bistriflate complexes.
中文翻译:

钼亚氨基亚烷基N-杂环卡宾配合物开环复分解聚合反应中电子选择性的起源
对立体选择性的理解和控制是开环易位聚合 (ROMP) 的核心方面。在此,我们报告了降冰片-2-烯 (NBE) 与 Mo( N -2,6-Me 2 -C 6 H 3 )(CHCMe 3 )(IMes)的E选择性 ROMP反应机理的详细量子化学研究(OTf) 2 ( 1 , IMes = 1,3-dimesitylimidazol-2-ylidene) 作为立体选择性聚合的第一步。基于一个烯四个不同的反应途径合成或烯抗NBE的任接近顺-或反已经研究了中性预催化剂的异构体。与最近建立的与末端烯烃的缔合机制形成对比,其中形成中性烯烃加合物,NBE 通过 [2 + 2] 环加成直接与催化剂反应形成钼环丁烷,反应势垒比游离态低约 30 kJ mol –1能量而不是通过形成催化剂 - 单体加合物。然而,NBE 的直接环加成仅在四种立体异构体中被发现。我们的研究结果强烈表明,这种立体选择性方法是E选择性的原因,并指向与中性 Mo 亚胺亚烷基N-杂环卡宾双氟甲磺酸酯配合物的烯烃复分解反应中的底物特异性反应机制。
更新日期:2021-08-09
中文翻译:

钼亚氨基亚烷基N-杂环卡宾配合物开环复分解聚合反应中电子选择性的起源
对立体选择性的理解和控制是开环易位聚合 (ROMP) 的核心方面。在此,我们报告了降冰片-2-烯 (NBE) 与 Mo( N -2,6-Me 2 -C 6 H 3 )(CHCMe 3 )(IMes)的E选择性 ROMP反应机理的详细量子化学研究(OTf) 2 ( 1 , IMes = 1,3-dimesitylimidazol-2-ylidene) 作为立体选择性聚合的第一步。基于一个烯四个不同的反应途径合成或烯抗NBE的任接近顺-或反已经研究了中性预催化剂的异构体。与最近建立的与末端烯烃的缔合机制形成对比,其中形成中性烯烃加合物,NBE 通过 [2 + 2] 环加成直接与催化剂反应形成钼环丁烷,反应势垒比游离态低约 30 kJ mol –1能量而不是通过形成催化剂 - 单体加合物。然而,NBE 的直接环加成仅在四种立体异构体中被发现。我们的研究结果强烈表明,这种立体选择性方法是E选择性的原因,并指向与中性 Mo 亚胺亚烷基N-杂环卡宾双氟甲磺酸酯配合物的烯烃复分解反应中的底物特异性反应机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号