当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular interactions play key role in stabilization of pHLIP at acidic conditions
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2021-07-09 , DOI: 10.1002/jcc.26719 Nicolas Frazee 1 , Blake Mertz 2
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2021-07-09 , DOI: 10.1002/jcc.26719 Nicolas Frazee 1 , Blake Mertz 2
Affiliation
|
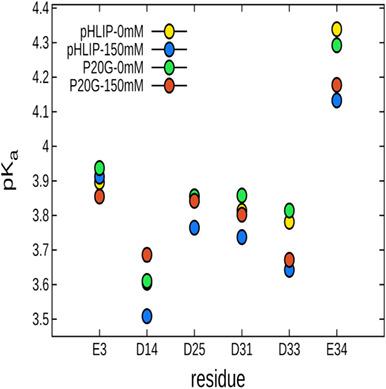
The pH-Low Insertion Peptide (pHLIP) is a membrane-active peptide that spontaneously folds into a transmembrane α-helix upon acidification. This activity enables pHLIP to potentially act as a vector for drugs related to diseases characterized by acidosis such as cancer or heart ischemia. Presently, due to aggregation-based effects, formulations of pHLIP are only viable at near-μM concentrations. In addition, since most of pHLIP's measurable qualities involve a membrane, probing the details of pHLIP in the interstitial region is difficult. In attempts to shed light on these issues, we performed constant pH molecular dynamics simulations on pHLIP as well as P20G, a variant with increased helicity, in solution at 0 and 150 mM NaCl over a broad range of pHs. In general, the addition of ions reduced the effective pKa of the acidic residues in pHLIP. P20G exhibits a higher helicity than pHLIP in general and is more compact than pHLIP at pH values under 4. In terms of charge effects, sodium cations localized predominantly to the C-terminus of the peptide with a high density of acidic residues. Additionally, the salt bridge between R11 and D14 is by far the most favored and particularly so with pHLIP at 150 mM NaCl. We expect that this approach will be a valuable tool to screen variants of pHLIP for favorable properties in solution, an aspect of pHLIP design that to this point has largely been neglected.
中文翻译:

分子内相互作用在酸性条件下稳定 pHLIP 中起关键作用
低 pH 插入肽 (pHLIP) 是一种膜活性肽,可自发折叠成跨膜α-酸化后的螺旋。这种活性使 pHLIP 有可能成为与以酸中毒为特征的疾病(如癌症或心脏缺血)相关的药物的载体。目前,由于基于聚集的效应,pHLIP 制剂仅在接近 μM 浓度下可行。此外,由于 pHLIP 的大多数可测量质量都涉及膜,因此很难在间隙区域探测 pHLIP 的细节。为了阐明这些问题,我们对 pHLIP 和 P20G(一种螺旋度增加的变体)在 0 和 150 mM NaCl 溶液中的广泛 pH 值进行了恒定 pH 分子动力学模拟。一般来说,离子的加入会降低有效的 pK apHLIP 中的酸性残基。P20G 通常比 pHLIP 表现出更高的螺旋度,并且在 pH 值低于 4 时比 pHLIP 更紧凑。就电荷效应而言,钠阳离子主要定位于具有高密度酸性残基的肽的 C 末端。此外,到目前为止,R11 和 D14 之间的盐桥是最受青睐的,尤其是在 150 mM NaCl 的 pHLIP 中。我们预计这种方法将成为筛选 pHLIP 变体以获得溶液中有利特性的有价值的工具,到目前为止,pHLIP 设计的一个方面在很大程度上被忽略了。
更新日期:2021-08-07
中文翻译:

分子内相互作用在酸性条件下稳定 pHLIP 中起关键作用
低 pH 插入肽 (pHLIP) 是一种膜活性肽,可自发折叠成跨膜α-酸化后的螺旋。这种活性使 pHLIP 有可能成为与以酸中毒为特征的疾病(如癌症或心脏缺血)相关的药物的载体。目前,由于基于聚集的效应,pHLIP 制剂仅在接近 μM 浓度下可行。此外,由于 pHLIP 的大多数可测量质量都涉及膜,因此很难在间隙区域探测 pHLIP 的细节。为了阐明这些问题,我们对 pHLIP 和 P20G(一种螺旋度增加的变体)在 0 和 150 mM NaCl 溶液中的广泛 pH 值进行了恒定 pH 分子动力学模拟。一般来说,离子的加入会降低有效的 pK apHLIP 中的酸性残基。P20G 通常比 pHLIP 表现出更高的螺旋度,并且在 pH 值低于 4 时比 pHLIP 更紧凑。就电荷效应而言,钠阳离子主要定位于具有高密度酸性残基的肽的 C 末端。此外,到目前为止,R11 和 D14 之间的盐桥是最受青睐的,尤其是在 150 mM NaCl 的 pHLIP 中。我们预计这种方法将成为筛选 pHLIP 变体以获得溶液中有利特性的有价值的工具,到目前为止,pHLIP 设计的一个方面在很大程度上被忽略了。











































 京公网安备 11010802027423号
京公网安备 11010802027423号