当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Antimicrobial Activity of the Glycocin Sublancin Is Dependent on an Active Phosphoenolpyruvate-Sugar Phosphotransferase System
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-07-09 , DOI: 10.1021/acsinfecdis.1c00157 Subhanip Biswas , Chunyu Wu , Wilfred A. van der Donk
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-07-09 , DOI: 10.1021/acsinfecdis.1c00157 Subhanip Biswas , Chunyu Wu , Wilfred A. van der Donk
|
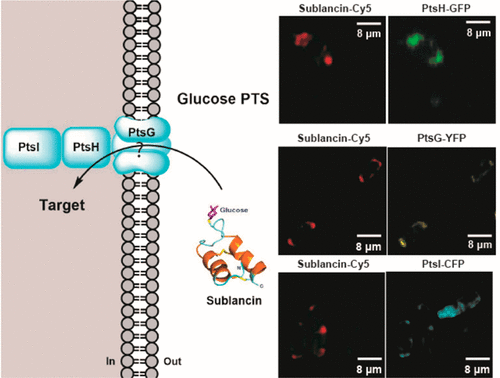
Antimicrobial resistance is a global challenge that is compounded by the limited number of available targets. Glycocins are antimicrobial glycopeptides that are believed to have novel targets. Previous studies have shown that the mechanism of action of the glycocin sublancin 168 involves the glucose uptake system. The phosphoenolpyruvate:sugar phosphotransferase system (PTS) phosphorylates the C6 hydroxyl group on glucose during import. Since sublancin carries a glucose on a Cys on an exposed loop, we investigated whether phosphorylation of this glucose might be involved in its mechanism of action by replacement with xylose. Surprisingly, the xylose analog was more active than wild-type sublancin and still required the glucose PTS for activity. Overexpression of the individual components of the PTS rendered cells more sensitive to sublancin, and their resistance frequency was considerably decreased. These observations suggest that sublancin is activated in some form by the glucose PTS or that sublancin imparts a deleterious gain-of-function on the PTS. Superresolution microscopy studies with fluorescent sublancin and fluorescently labeled PTS proteins revealed localization of both at the poles of cells. Resistant mutants raised under conditions that would minimize mutation of the PTS revealed mutations in FliQ, a protein involved in the flagellar protein export process. Overexpression of FliQ lead to decreased sensitivity of cells to sublancin. Collectively, these findings enforce a model in which the PTS is required for sublancin activity, either by inducing a deleterious gain-of-function or by activating or transporting sublancin.
中文翻译:

Glycocin Sublancin 的抗菌活性依赖于活性磷酸烯醇丙酮酸-糖磷酸转移酶系统
抗菌素耐药性是一项全球性挑战,由于可用目标数量有限而更加复杂。甘氨酸是抗菌糖肽,被认为具有新的靶点。先前的研究表明,甘氨酸 sublancin 168 的作用机制涉及葡萄糖摄取系统。磷酸烯醇丙酮酸:糖磷酸转移酶系统 (PTS) 在输入过程中磷酸化葡萄糖上的 C6 羟基。由于 sublancin 在暴露环上的 Cys 上携带葡萄糖,我们研究了这种葡萄糖的磷酸化是否可能通过木糖替代参与其作用机制。令人惊讶的是,木糖类似物比野生型 sublancin 更具活性,并且仍然需要葡萄糖 PTS 才能发挥活性。PTS 各个成分的过度表达使细胞对 sublancin 更敏感,并且它们的电阻频率大大降低。这些观察结果表明,sublancin 以某种形式被葡萄糖 PTS 激活,或者 sublancin 对 PTS 产生了有害的功能增益。用荧光 sublancin 和荧光标记的 PTS 蛋白进行的超分辨率显微镜研究揭示了两者都位于细胞的两极。在将 PTS 突变降至最低的条件下培养的抗性突变体揭示了 FliQ 中的突变,这是一种参与鞭毛蛋白输出过程的蛋白质。FliQ 的过表达导致细胞对 sublancin 的敏感性降低。总的来说,这些发现强化了一个模型,其中 PTS 是 sublancin 活性所必需的,要么通过诱导有害的功能获得,要么通过激活或运输 sublancin。这些观察结果表明,sublancin 以某种形式被葡萄糖 PTS 激活,或者 sublancin 对 PTS 产生了有害的功能增益。用荧光 sublancin 和荧光标记的 PTS 蛋白进行的超分辨率显微镜研究揭示了两者都位于细胞的两极。在将 PTS 突变降至最低的条件下培养的抗性突变体揭示了 FliQ 中的突变,这是一种参与鞭毛蛋白输出过程的蛋白质。FliQ 的过表达导致细胞对 sublancin 的敏感性降低。总的来说,这些发现强化了一个模型,其中 PTS 是 sublancin 活性所必需的,要么通过诱导有害的功能获得,要么通过激活或运输 sublancin。这些观察结果表明,sublancin 以某种形式被葡萄糖 PTS 激活,或者 sublancin 对 PTS 产生了有害的功能增益。用荧光 sublancin 和荧光标记的 PTS 蛋白进行的超分辨率显微镜研究揭示了两者都位于细胞的两极。在将 PTS 突变降至最低的条件下培养的抗性突变体揭示了 FliQ 中的突变,这是一种参与鞭毛蛋白输出过程的蛋白质。FliQ 的过表达导致细胞对 sublancin 的敏感性降低。总的来说,这些发现强化了一个模型,其中 PTS 是 sublancin 活性所必需的,要么通过诱导有害的功能获得,要么通过激活或运输 sublancin。
更新日期:2021-08-13
中文翻译:

Glycocin Sublancin 的抗菌活性依赖于活性磷酸烯醇丙酮酸-糖磷酸转移酶系统
抗菌素耐药性是一项全球性挑战,由于可用目标数量有限而更加复杂。甘氨酸是抗菌糖肽,被认为具有新的靶点。先前的研究表明,甘氨酸 sublancin 168 的作用机制涉及葡萄糖摄取系统。磷酸烯醇丙酮酸:糖磷酸转移酶系统 (PTS) 在输入过程中磷酸化葡萄糖上的 C6 羟基。由于 sublancin 在暴露环上的 Cys 上携带葡萄糖,我们研究了这种葡萄糖的磷酸化是否可能通过木糖替代参与其作用机制。令人惊讶的是,木糖类似物比野生型 sublancin 更具活性,并且仍然需要葡萄糖 PTS 才能发挥活性。PTS 各个成分的过度表达使细胞对 sublancin 更敏感,并且它们的电阻频率大大降低。这些观察结果表明,sublancin 以某种形式被葡萄糖 PTS 激活,或者 sublancin 对 PTS 产生了有害的功能增益。用荧光 sublancin 和荧光标记的 PTS 蛋白进行的超分辨率显微镜研究揭示了两者都位于细胞的两极。在将 PTS 突变降至最低的条件下培养的抗性突变体揭示了 FliQ 中的突变,这是一种参与鞭毛蛋白输出过程的蛋白质。FliQ 的过表达导致细胞对 sublancin 的敏感性降低。总的来说,这些发现强化了一个模型,其中 PTS 是 sublancin 活性所必需的,要么通过诱导有害的功能获得,要么通过激活或运输 sublancin。这些观察结果表明,sublancin 以某种形式被葡萄糖 PTS 激活,或者 sublancin 对 PTS 产生了有害的功能增益。用荧光 sublancin 和荧光标记的 PTS 蛋白进行的超分辨率显微镜研究揭示了两者都位于细胞的两极。在将 PTS 突变降至最低的条件下培养的抗性突变体揭示了 FliQ 中的突变,这是一种参与鞭毛蛋白输出过程的蛋白质。FliQ 的过表达导致细胞对 sublancin 的敏感性降低。总的来说,这些发现强化了一个模型,其中 PTS 是 sublancin 活性所必需的,要么通过诱导有害的功能获得,要么通过激活或运输 sublancin。这些观察结果表明,sublancin 以某种形式被葡萄糖 PTS 激活,或者 sublancin 对 PTS 产生了有害的功能增益。用荧光 sublancin 和荧光标记的 PTS 蛋白进行的超分辨率显微镜研究揭示了两者都位于细胞的两极。在将 PTS 突变降至最低的条件下培养的抗性突变体揭示了 FliQ 中的突变,这是一种参与鞭毛蛋白输出过程的蛋白质。FliQ 的过表达导致细胞对 sublancin 的敏感性降低。总的来说,这些发现强化了一个模型,其中 PTS 是 sublancin 活性所必需的,要么通过诱导有害的功能获得,要么通过激活或运输 sublancin。











































 京公网安备 11010802027423号
京公网安备 11010802027423号