当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strengthening the CAR-T cell therapeutic application using CRISPR/Cas9 technology
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-07-09 , DOI: 10.1002/bit.27882 Muhammad Sadeqi Nezhad 1 , Mahboubeh Yazdanifar 2 , Meghdad Abdollahpour-Alitappeh 3 , Arash Sattari 4 , Alexander Seifalian 5 , Nader Bagheri 6
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-07-09 , DOI: 10.1002/bit.27882 Muhammad Sadeqi Nezhad 1 , Mahboubeh Yazdanifar 2 , Meghdad Abdollahpour-Alitappeh 3 , Arash Sattari 4 , Alexander Seifalian 5 , Nader Bagheri 6
Affiliation
|
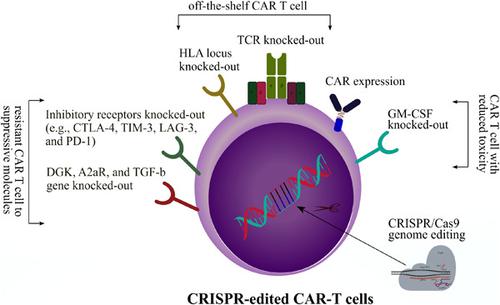
Adoptive cell immunotherapy with chimeric antigen receptor T (CAR-T) cell has brought a revolutionary means of treatment for aggressive diseases such as hematologic malignancies and solid tumors. Over the last decade, the United States Food and Drug Administration (FDA) approved five types of CAR-T cell therapies for hematologic malignancies, including Idecabtagene vicleucel (Abecma), Lisocabtagene maraleucel (Breyanzi), Brexucabtagene autoleucel (Tecartus), Tisagenlecleucel (Kymriah), and Axicabtagene ciloleucel (Yescarta). Despite outstanding results gained from different clinical trials, CAR-T cell therapy is not free from side effects and toxicities, and needs careful investigations and improvements. Gene-editing technology, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system, has emerged as a promising tool to address some of the CAR-T therapy hurdles. Using CRISPR/Cas9 technology, CAR expression as well as other cellular pathways can be modified in various ways to enhance CAR-T cells antitumor function and persistence in immunosuppressive tumor microenvironment. CRISPR/Cas9 technology can also be used to decrease CAR-T cell toxicities and side effects. Hereby, we discussed the practical challenges and hurdles related to the accuracy, efficiency, efficacy, safety, and delivery of CRISPR/Cas9 technology to the genetically engineered-T cells. Combining of these two state-of-the-art technologies, CRISPR/Cas9 and CAR-T cells, the field of oncology has an extraordinary opportunity to enter a new era of immunotherapy, which offers novel therapeutic options for different types of tumors.
中文翻译:

利用 CRISPR/Cas9 技术加强 CAR-T 细胞治疗应用
嵌合抗原受体 T (CAR-T) 细胞的过继性细胞免疫疗法为恶性血液病和实体瘤等侵袭性疾病带来了革命性的治疗手段。过去十年,美国食品药品监督管理局(FDA)批准了五种用于血液系统恶性肿瘤的 CAR-T 细胞疗法,包括 Idecabtagene vicleucel(Abecma)、Lisocabtagene maraleucel(Breyanzi)、Brexucabtagene autoleucel(Tecartus)、Tisagenlecleucel(Kymriah) ) 和 Axicabtagene ciloleucel (Yescarta)。尽管从不同的临床试验中获得了显着的结果,但 CAR-T 细胞疗法并非没有副作用和毒性,需要仔细研究和改进。基因编辑技术,成簇的规则间隔短回文重复序列 (CRISPR)/CRISPR 相关蛋白 9 (Cas9) 系统,已成为解决一些 CAR-T 治疗障碍的有前途的工具。使用 CRISPR/Cas9 技术,可以通过各种方式修改 CAR 表达以及其他细胞途径,以增强 CAR-T 细胞的抗肿瘤功能和在免疫抑制性肿瘤微环境中的持久性。CRISPR/Cas9 技术还可用于降低 CAR-T 细胞毒性和副作用。因此,我们讨论了与 CRISPR/Cas9 技术向基因工程 T 细胞的准确性、效率、功效、安全性和传递相关的实际挑战和障碍。结合这两种最先进的技术,CRISPR/Cas9 和 CAR-T 细胞,肿瘤学领域拥有进入免疫治疗新时代的绝佳机会,为不同类型的肿瘤提供新的治疗选择。
更新日期:2021-09-12
中文翻译:

利用 CRISPR/Cas9 技术加强 CAR-T 细胞治疗应用
嵌合抗原受体 T (CAR-T) 细胞的过继性细胞免疫疗法为恶性血液病和实体瘤等侵袭性疾病带来了革命性的治疗手段。过去十年,美国食品药品监督管理局(FDA)批准了五种用于血液系统恶性肿瘤的 CAR-T 细胞疗法,包括 Idecabtagene vicleucel(Abecma)、Lisocabtagene maraleucel(Breyanzi)、Brexucabtagene autoleucel(Tecartus)、Tisagenlecleucel(Kymriah) ) 和 Axicabtagene ciloleucel (Yescarta)。尽管从不同的临床试验中获得了显着的结果,但 CAR-T 细胞疗法并非没有副作用和毒性,需要仔细研究和改进。基因编辑技术,成簇的规则间隔短回文重复序列 (CRISPR)/CRISPR 相关蛋白 9 (Cas9) 系统,已成为解决一些 CAR-T 治疗障碍的有前途的工具。使用 CRISPR/Cas9 技术,可以通过各种方式修改 CAR 表达以及其他细胞途径,以增强 CAR-T 细胞的抗肿瘤功能和在免疫抑制性肿瘤微环境中的持久性。CRISPR/Cas9 技术还可用于降低 CAR-T 细胞毒性和副作用。因此,我们讨论了与 CRISPR/Cas9 技术向基因工程 T 细胞的准确性、效率、功效、安全性和传递相关的实际挑战和障碍。结合这两种最先进的技术,CRISPR/Cas9 和 CAR-T 细胞,肿瘤学领域拥有进入免疫治疗新时代的绝佳机会,为不同类型的肿瘤提供新的治疗选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号