当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arylquinolinecarboxamides: Synthesis, in vitro and in silico studies against Mycobacterium tuberculosis
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-07-08 , DOI: 10.1002/jhet.4340 Fostino R. B. Bokosi 1 , Richard M. Beteck 1, 2 , Audrey Jordaan 3 , Ronnet Seldon 4 , Digby F. Warner 3, 5 , Tendamudzimu Tshiwawa 1 , Kevin Lobb 1 , Setshaba D. Khanye 1, 6, 7
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-07-08 , DOI: 10.1002/jhet.4340 Fostino R. B. Bokosi 1 , Richard M. Beteck 1, 2 , Audrey Jordaan 3 , Ronnet Seldon 4 , Digby F. Warner 3, 5 , Tendamudzimu Tshiwawa 1 , Kevin Lobb 1 , Setshaba D. Khanye 1, 6, 7
Affiliation
|
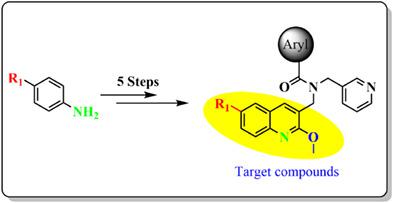
A series of fourteen 6-substituted-2-(methoxyquinolin-3-yl) methyl)-N-(pyridin-3-ylmethyl) benzamides was prepared from commercially available anilines in five simple and convenient synthetic steps. The structures of all new products were confirmed by routine spectroscopic methods: IR, 1H and 13C NMR, and HRMS (electrospray ionization). The resulting arylquinolinecarboxamides were subjected to biological screening assay for in vitro inhibitory activity against Mycobacterium tuberculosis (Mtb) H37Rv strain. Several compounds exhibited modest antitubercular activity with compounds 8–11, 15 and 19 exhibiting MIC90 values in the range of 32–85 μM. The antitubercular data suggested that inhibition of Mtb can be imparted by the introduction of a non-polar substituent on C-6 of the quinoline scaffold. Further, to understand the possible mode of action of the series, the reported compounds and bedaquiline were subjected to in silico docking studies against MtbATPase to determine their potential to interfere with the mycobacterial adenosine triphosphate (ATP) synthase. The results showed that these compounds have the potential to serve as antimycobacterial agents. In silico ADME pharmacokinetic prediction results showed the ability of these arylquinolinecarcboxamides to be absorbed, distributed, metabolized and excreted efficiently.
中文翻译:

芳基喹啉甲酰胺:针对结核分枝杆菌的合成、体外和计算机研究
一系列十四个 6-取代-2-(甲氧基喹啉-3-基)甲基) -N- (吡啶-3-基甲基)苯甲酰胺通过五个简单方便的合成步骤从市售苯胺制备。所有新产品的结构均通过常规光谱方法确认:IR、1 H 和13 C NMR 以及 HRMS(电喷雾电离)。对所得的芳基喹啉甲酰胺进行生物筛选测定,以确定对结核分枝杆菌( Mtb ) H37Rv 菌株的体外抑制活性。几个化合物显示出适度的抗结核活性用化合物8-11,15和19MIC 90值在 32–85 μM 范围内。抗结核数据表明可以通过在喹啉支架的 C-6 上引入非极性取代基来抑制Mtb。此外,为了了解该系列的可能作用方式,对报告的化合物和贝达喹啉进行了针对Mtb ATPase 的计算机对接研究,以确定它们干扰分枝杆菌三磷酸腺苷 (ATP) 合酶的潜力。结果表明,这些化合物具有作为抗分枝杆菌剂的潜力。电脑模拟 ADME药代动力学预测结果表明,这些芳基喹啉甲酰胺具有高效吸收、分布、代谢和排泄的能力。
更新日期:2021-07-08
中文翻译:

芳基喹啉甲酰胺:针对结核分枝杆菌的合成、体外和计算机研究
一系列十四个 6-取代-2-(甲氧基喹啉-3-基)甲基) -N- (吡啶-3-基甲基)苯甲酰胺通过五个简单方便的合成步骤从市售苯胺制备。所有新产品的结构均通过常规光谱方法确认:IR、1 H 和13 C NMR 以及 HRMS(电喷雾电离)。对所得的芳基喹啉甲酰胺进行生物筛选测定,以确定对结核分枝杆菌( Mtb ) H37Rv 菌株的体外抑制活性。几个化合物显示出适度的抗结核活性用化合物8-11,15和19MIC 90值在 32–85 μM 范围内。抗结核数据表明可以通过在喹啉支架的 C-6 上引入非极性取代基来抑制Mtb。此外,为了了解该系列的可能作用方式,对报告的化合物和贝达喹啉进行了针对Mtb ATPase 的计算机对接研究,以确定它们干扰分枝杆菌三磷酸腺苷 (ATP) 合酶的潜力。结果表明,这些化合物具有作为抗分枝杆菌剂的潜力。电脑模拟 ADME药代动力学预测结果表明,这些芳基喹啉甲酰胺具有高效吸收、分布、代谢和排泄的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号