当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trifunctionalization of Aryl Iodides with Two Distinct Nitrogen and Carbon Electrophiles by Palladium/Norbornene Catalysis
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2021-07-08 , DOI: 10.1002/cjoc.202100467 Jing Wang 1 , Hui Wang 1 , Zihan Wang 1 , Linqiang Li 1 , Cheng Qin 1 , Xinjun Luan 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2021-07-08 , DOI: 10.1002/cjoc.202100467 Jing Wang 1 , Hui Wang 1 , Zihan Wang 1 , Linqiang Li 1 , Cheng Qin 1 , Xinjun Luan 1
Affiliation
|
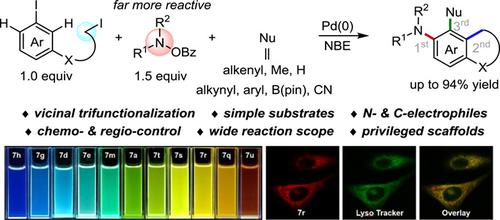
Herein, we report a highly chemo- and regioselective vicinal trifunctionalization of aryl iodides by palladium/norbornene (Pd/NBE) catalysis. The key feature of this new method is the introduction of two distinct nitrogen and carbon electrophiles, with a large gap in reactivity, for ortho-unsubstituted aryl iodides via an intermolecular and intramolecular C―H functionalization, respectively. Eight types of ipso terminations can be coupled with both ortho-amination and ortho-alkylation, affording a variety of polysubstituted benzoheterocyclic scaffolds. Silicon-tethered substrates can lead to polyfucntional arenes via a single-step operation. Noteworthy, these products exhibit full-color-tunable strong fluorescence emissions with large Stokes shifts, and product 7r can serve as a fluorescent probe to specifically target lysosome in living cells.
中文翻译:

钯/降冰片烯催化具有两种不同的氮和碳亲电试剂的芳基碘三官能化
在此,我们报告了通过钯/降冰片烯 (Pd/NBE) 催化对芳基碘化物进行高度化学和区域选择性的邻位三官能化。这种新方法的关键特征是引入了两个不同的氮和碳亲电子的,在反应性有很大的差距,对邻-未被取代的芳基碘经由分别的分子内和分子CA€•ħ官能化,。八种类型的本位终端可以与两个邻位-amination和邻烷基化,得到多种多取代的苯并杂环支架。硅系衬底可以通过以下方式产生多官能芳烃单步操作。值得注意的是,这些产品表现出具有大斯托克斯位移的全色可调强荧光发射,并且产品 7r 可以作为荧光探针来特异性靶向活细胞中的溶酶体。
更新日期:2021-09-15

中文翻译:

钯/降冰片烯催化具有两种不同的氮和碳亲电试剂的芳基碘三官能化
在此,我们报告了通过钯/降冰片烯 (Pd/NBE) 催化对芳基碘化物进行高度化学和区域选择性的邻位三官能化。这种新方法的关键特征是引入了两个不同的氮和碳亲电子的,在反应性有很大的差距,对邻-未被取代的芳基碘经由分别的分子内和分子CA€•ħ官能化,。八种类型的本位终端可以与两个邻位-amination和邻烷基化,得到多种多取代的苯并杂环支架。硅系衬底可以通过以下方式产生多官能芳烃单步操作。值得注意的是,这些产品表现出具有大斯托克斯位移的全色可调强荧光发射,并且产品 7r 可以作为荧光探针来特异性靶向活细胞中的溶酶体。




























 京公网安备 11010802027423号
京公网安备 11010802027423号