Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Domino Aminocarbonylation of Alkynols: Direct and Selective Synthesis of Itaconimides
JACS Au ( IF 8.5 ) Pub Date : 2021-07-09 , DOI: 10.1021/jacsau.1c00221 Yao Ge 1 , Fei Ye 1, 2 , Ji Yang 1 , Anke Spannenberg 1 , Ralf Jackstell 1 , Matthias Beller 1
JACS Au ( IF 8.5 ) Pub Date : 2021-07-09 , DOI: 10.1021/jacsau.1c00221 Yao Ge 1 , Fei Ye 1, 2 , Ji Yang 1 , Anke Spannenberg 1 , Ralf Jackstell 1 , Matthias Beller 1
Affiliation
|
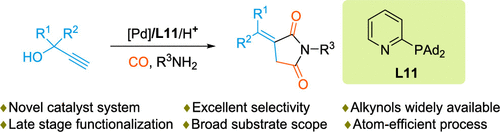
The first direct and selective synthesis of substituted itaconimdes by palladium-catalyzed aminocarbonylation of alkynols is reported. Key to the success of this transformation is the use of a novel catalyst system involving ligand L11 and appropriate reaction conditions. In the protocol here presented, easily available propargylic alcohols react with N-nucleophiles including aryl- and alkylamines as well as aryl hydrazines to provide a broad variety of interesting heterocycles with high catalyst activity and excellent selectivity. The synthetic utility of the protocol is demonstrated in the synthesis of natural product 11 with aminocarbonylation as the key step. Mechanistic studies and control experiments reveal the crucial role of the hydroxyl group in the substrate for the control of selectivity.
中文翻译:

炔醇的钯催化多米诺氨基羰基化:衣康酰亚胺的直接和选择性合成
报道了第一个通过钯催化炔醇氨基羰基化直接和选择性合成取代衣康酰亚胺。这种转变成功的关键是使用一种新型催化剂体系,包括配体L11和适当的反应条件。在此处介绍的协议中,容易获得的炔丙醇与N-亲核试剂(包括芳基胺和烷基胺以及芳基肼)反应,以提供各种具有高催化剂活性和出色选择性的有趣杂环。该协议的合成效用在天然产物11的合成中得到证明以氨基羰基化为关键步骤。机理研究和控制实验揭示了底物中羟基在控制选择性方面的关键作用。
更新日期:2021-08-23
中文翻译:

炔醇的钯催化多米诺氨基羰基化:衣康酰亚胺的直接和选择性合成
报道了第一个通过钯催化炔醇氨基羰基化直接和选择性合成取代衣康酰亚胺。这种转变成功的关键是使用一种新型催化剂体系,包括配体L11和适当的反应条件。在此处介绍的协议中,容易获得的炔丙醇与N-亲核试剂(包括芳基胺和烷基胺以及芳基肼)反应,以提供各种具有高催化剂活性和出色选择性的有趣杂环。该协议的合成效用在天然产物11的合成中得到证明以氨基羰基化为关键步骤。机理研究和控制实验揭示了底物中羟基在控制选择性方面的关键作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号