Biochemistry (Moscow) ( IF 2.3 ) Pub Date : 2021-07-08 , DOI: 10.1134/s0006297921070105 Hamed Ghadiri 1 , Sana Alavi 2 , Bahareh Dabirmanesh 1 , Khosro Khajeh 1, 2
|
Abstract
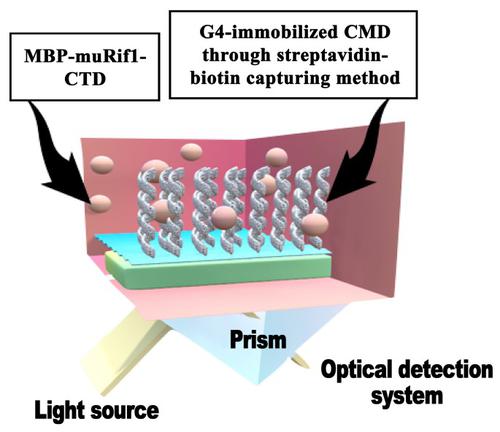
One of the main players in the cell-specific replication timing pattern is Rap1 interacting factor-1 (Rif1). Rif1 protein consists of N-terminal and C-terminal domains and an intrinsically disordered region in between. It has been suggested that both N- and C-termini of Rif1 are capable of binding to DNA with particularly high affinity to cruciform DNA structures. In the present study, we expressed, solubilized, and purified the maltose-binding protein-tagged murine Rif1 C-terminal domain (MBP-muRif1-CTD). Biological activity of the purified protein was assessed by the electrophoretic mobility shift assay (EMSA) and surface plasmon resonance (SPR). Our results show that the MBP-muRif1-CTD binds G-quadruplex (G4) structure with high affinity (KD 19.0 ± 0.8 nM), as was previously suggested. This study is the first step in investigation of the interaction of MBP-Profinity eXact-muRif1-CTD and G4 by SPR.
Graphic abstract
中文翻译:

通过 SPR 表征 MBP 标记的 MuRif1-C-末端结构域与 G-四链体 DNA 的相互作用
摘要
细胞特异性复制时间模式中的主要参与者之一是 Rap1 相互作用因子-1 (Rif1)。Rif1 蛋白由 N 端和 C 端结构域以及中间的内在无序区域组成。已经表明 Rif1 的 N 端和 C 端都能够与 DNA 结合,对十字形 DNA 结构具有特别高的亲和力。在本研究中,我们表达、溶解和纯化了麦芽糖结合蛋白标记的鼠 Rif1 C 末端结构域 (MBP-muRif1-CTD)。通过电泳迁移率变化测定 (EMSA) 和表面等离子体共振 (SPR) 评估纯化蛋白质的生物活性。我们的结果表明,MBP-muRif1-CTD 以高亲和力(K D19.0 ± 0.8 nM),如之前所建议的。本研究是 SPR 研究 MBP-Profinity eXact-muRif1-CTD 和 G4 相互作用的第一步。









































 京公网安备 11010802027423号
京公网安备 11010802027423号