Giant ( IF 5.4 ) Pub Date : 2021-07-07 , DOI: 10.1016/j.giant.2021.100071 Zhixiong Deng 1 , Xin You 1 , Bing Yuan 1, 2 , Kai Yang 1, 2
|
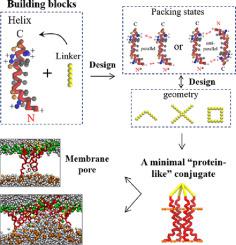
Biomimetic membrane nanopores have great potentials for bio- and nanotechnology applications. Here, a design strategy of an artificial protein by reasonably conjugating peptides and polymers for membrane poration was developed based on the α-helical bundle framework. A serial of peptide-polymer conjugates, composed of the α-helical peptide melittin (Mel) and clinically available polymer polyethylene glycol, were fabricated. Within them, the S-Mel, consisting of four helices arranged in a star-like bundle structure, demonstrated the most improved performance. Specifically, one single S-Mel molecule can build a stable membrane pore. Moreover, the obtained pores display stimuli-responsive properties in both size (including water flux) and structure, which is ascribed to the molecular architecture-regulated helical packing changes of S-Mel during membrane actions. These results promise S-Mel the ability to achieve ON OFF switching and gating effects for selective mass translocation across a cell membrane and help understand the structure-function relationship of proteins.
OFF switching and gating effects for selective mass translocation across a cell membrane and help understand the structure-function relationship of proteins.
中文翻译:

用于有效膜穿孔的最小“类蛋白质”缀合物的计算设计
仿生膜纳米孔在生物和纳米技术应用方面具有巨大潜力。在这里,基于α-螺旋束框架开发了一种通过合理结合肽和聚合物用于膜穿孔的人工蛋白质的设计策略。制造了一系列由 α-螺旋肽蜂毒肽 (Mel) 和临床上可用的聚合物聚乙二醇组成的肽-聚合物偶联物。在它们中,由排列成星状束结构的四个螺旋组成的 S-Mel 表现出最大的改进性能。具体来说,一个单一的 S-Mel 分子可以构建一个稳定的膜孔。此外,获得的孔在大小(包括水通量)和结构上都显示出刺激响应特性,这归因于膜作用期间 S-Mel 的分子结构调节的螺旋堆积变化。这些结果保证了 S-Mel 能够实现 ON 关闭开关和门控效应,用于跨细胞膜的选择性质量易位,有助于了解蛋白质的结构-功能关系。
关闭开关和门控效应,用于跨细胞膜的选择性质量易位,有助于了解蛋白质的结构-功能关系。









































 京公网安备 11010802027423号
京公网安备 11010802027423号