Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2021-07-07 , DOI: 10.1016/j.xcrp.2021.100490 En Li 1, 2 , Qian Wang 1, 2 , Yuxing Cai 1, 2 , Jiean Chen 2, 3 , Yong Huang 4
|
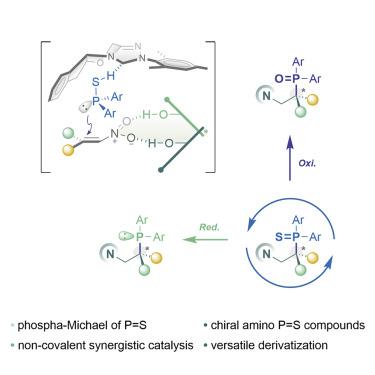
Despite rapid advances in phosphine-containing compound as a privileged ligand for transition metal catalysis, synthesis and coordination chemistry are underexplored for the phosphine sulfide analogs. In this article, we report the enantioselective phospha-Michael addition reaction that leads to α-chiral phosphine sulfide. An independent hydrogen-bonding co-catalyst is found essential to high enantioselectivity (up to 98% yield and 95% enantiomeric excess [ee]). Divergent derivatizations under mild conditions are also attempted for the generic phosphine and phosphine oxide compounds, further emphasizing the bridge role of phosphine sulfide. An X-ray diffraction structure of κ3-NNS palladium complex also authenticates the coordination ability of p = S moiety.
中文翻译:

非共价有机催化合成α-手性硫化膦
尽管在含膦化合物作为过渡金属催化的特权配体方面取得了快速进展,但对于硫化膦类似物的合成和配位化学仍未得到充分探索。在本文中,我们报告了导致 α-手性硫化膦的对映选择性磷酸-迈克尔加成反应。发现独立的氢键助催化剂对于高对映选择性(高达 98% 的产率和 95% 的对映体过量 [ee])必不可少。还尝试在温和条件下对通用膦和氧化膦化合物进行不同的衍生化,进一步强调了硫化膦的桥接作用。κ 3 -NNS 钯配合物的 X 射线衍射结构也验证了 p = S 部分的配位能力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号