当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cathodic Corrosion of Metal Electrodes—How to Prevent It in Electroorganic Synthesis
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-07-06 , DOI: 10.1021/acs.chemrev.1c00148 Tom Wirtanen 1 , Tobias Prenzel 1 , Jean-Philippe Tessonnier 2, 3 , Siegfried R Waldvogel 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-07-06 , DOI: 10.1021/acs.chemrev.1c00148 Tom Wirtanen 1 , Tobias Prenzel 1 , Jean-Philippe Tessonnier 2, 3 , Siegfried R Waldvogel 1
Affiliation
|
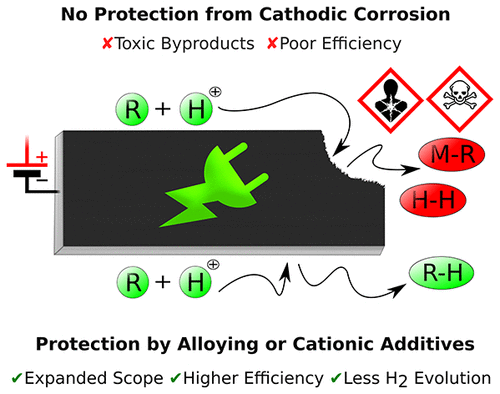
The critical aspects of the corrosion of metal electrodes in cathodic reductions are covered. We discuss the involved mechanisms including alloying with alkali metals, cathodic etching in aqueous and aprotic media, and formation of metal hydrides and organometallics. Successful approaches that have been implemented to suppress cathodic corrosion are reviewed. We present several examples from electroorganic synthesis where the clever use of alloys instead of soft neat heavy metals and the application of protective cationic additives have allowed to successfully exploit these materials as cathodes. Because of the high overpotential for the hydrogen evolution reaction, such cathodes can contribute toward more sustainable green synthetic processes. The reported strategies expand the applications of organic electrosynthesis because a more negative regime is accessible within protic media and common metal poisons, e.g., sulfur-containing substrates, are compatible with these cathodes. The strongly diminished hydrogen evolution side reaction paves the way for more efficient reductive electroorganic conversions.
中文翻译:

金属电极的阴极腐蚀——有机电合成中如何预防
涵盖了阴极还原中金属电极腐蚀的关键方面。我们讨论了涉及的机制,包括与碱金属合金化、水性和非质子介质中的阴极蚀刻以及金属氢化物和有机金属的形成。回顾了已实施以抑制阴极腐蚀的成功方法。我们展示了几个来自有机电合成的例子,其中巧妙地使用合金代替柔软的纯重金属以及保护性阳离子添加剂的应用,成功地将这些材料用作阴极。由于析氢反应的高过电位,这种阴极可以促进更可持续的绿色合成过程。报道的策略扩展了有机电合成的应用,因为在质子介质中可以获得更负的状态,并且常见的金属毒物,例如含硫底物,与这些阴极兼容。显着减少的析氢副反应为更有效的还原有机电转化铺平了道路。
更新日期:2021-09-08
中文翻译:

金属电极的阴极腐蚀——有机电合成中如何预防
涵盖了阴极还原中金属电极腐蚀的关键方面。我们讨论了涉及的机制,包括与碱金属合金化、水性和非质子介质中的阴极蚀刻以及金属氢化物和有机金属的形成。回顾了已实施以抑制阴极腐蚀的成功方法。我们展示了几个来自有机电合成的例子,其中巧妙地使用合金代替柔软的纯重金属以及保护性阳离子添加剂的应用,成功地将这些材料用作阴极。由于析氢反应的高过电位,这种阴极可以促进更可持续的绿色合成过程。报道的策略扩展了有机电合成的应用,因为在质子介质中可以获得更负的状态,并且常见的金属毒物,例如含硫底物,与这些阴极兼容。显着减少的析氢副反应为更有效的还原有机电转化铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号