当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Affinity Points of Interaction on Antibody Allow Synthesis of Stable and Highly Functional Antibody–Gold Nanoparticle Conjugates
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-07-06 , DOI: 10.1021/acs.bioconjchem.1c00261 Samuel Okyem 1 , Olatunde Awotunde 1 , Tosin Ogunlusi 1 , McKenzie B Riley 1 , Jeremy D Driskell 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-07-06 , DOI: 10.1021/acs.bioconjchem.1c00261 Samuel Okyem 1 , Olatunde Awotunde 1 , Tosin Ogunlusi 1 , McKenzie B Riley 1 , Jeremy D Driskell 1
Affiliation
|
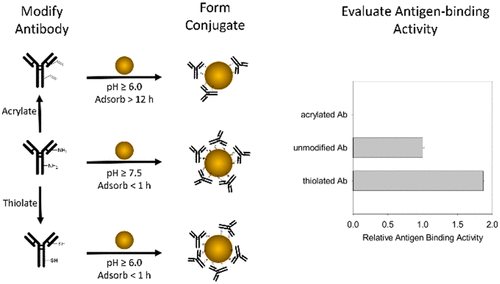
Many emerging nanobiotechnologies rely on the proper function of proteins immobilized on gold nanoparticles. Often, the surface chemistry of the AuNP is engineered to control the orientation, surface coverage, and structure of the adsorbed protein to maximize conjugate function. Here, we chemically modified antibody to investigate the effect of protein surface chemistries on adsorption to AuNPs. A monoclonal anti-horseradish peroxidase IgG antibody (anti-HRP) was reacted with N-succinimidyl acrylate (NSA) or reduced dithiobissuccinimidyl propionate (DSP) to modify lysine residues. Zeta potential measurements confirmed that both chemical modifications reduced the localized regions of positive charge on the protein surface, while the DSP modification incorporated additional free thiols. Dynamic light scattering confirmed that native and chemically modified antibodies adsorbed onto AuNPs to form bioconjugates; however, adsorption kinetics revealed that the NSA-modified antibody required significantly more time to allow for the formation of a hard corona. Moreover, conjugates formed with the NSA-modified antibody lost antigen-binding function, whereas unmodified and DSP-modified antibodies adsorbed onto AuNPs to form functional conjugates. These results indicate that high-affinity functional groups are required to prevent protein unfolding and loss of function when adsorbed on the AuNP surface. The reduced protein charge and high-affinity thiol groups on the DSP-modified antibody enabled pH-dependent control of protein orientation and the formation of highly active conjugates at solution pHs (<7.5) that are inaccessible with unmodified antibody due to conjugate aggregation. This study establishes parameters for protein modification to facilitate the formation of highly functional and stable protein–AuNP conjugates.
中文翻译:

抗体上的高亲和力相互作用点允许合成稳定且功能强大的抗体-金纳米粒子缀合物
许多新兴的纳米生物技术依赖于固定在金纳米粒子上的蛋白质的适当功能。通常,AuNP 的表面化学经过设计以控制吸附蛋白质的方向、表面覆盖率和结构,以最大限度地发挥共轭功能。在这里,我们化学修饰的抗体来研究蛋白质表面化学对 AuNPs 吸附的影响。将单克隆抗辣根过氧化物酶 IgG 抗体(抗 HRP)与丙烯酸 N-琥珀酰亚胺酯 (NSA) 或还原的二硫代双琥珀酰亚胺丙酸酯 (DSP) 反应以修饰赖氨酸残基。Zeta 电位测量证实,两种化学修饰都减少了蛋白质表面正电荷的局部区域,而 DSP 修饰结合了额外的游离硫醇。动态光散射证实天然和化学修饰的抗体吸附在 AuNP 上形成生物偶联物;然而,吸附动力学表明,NSA 修饰的抗体需要更多的时间来形成硬电晕。此外,与 NSA 修饰的抗体形成的结合物失去了抗原结合功能,而未修饰和 DSP 修饰的抗体吸附在 AuNP 上形成功能性结合物。这些结果表明,当吸附在 AuNP 表面时,需要高亲和力官能团来防止蛋白质解折叠和功能丧失。DSP 修饰抗体上减少的蛋白质电荷和高亲和力硫醇基团能够实现蛋白质方向的 pH 依赖性控制,并在溶液 pH 值 (<7. 5) 由于偶联物聚集,未经修饰的抗体无法获得。该研究建立了蛋白质修饰参数,以促进高功能和稳定的蛋白质-AuNP 偶联物的形成。
更新日期:2021-08-19
中文翻译:

抗体上的高亲和力相互作用点允许合成稳定且功能强大的抗体-金纳米粒子缀合物
许多新兴的纳米生物技术依赖于固定在金纳米粒子上的蛋白质的适当功能。通常,AuNP 的表面化学经过设计以控制吸附蛋白质的方向、表面覆盖率和结构,以最大限度地发挥共轭功能。在这里,我们化学修饰的抗体来研究蛋白质表面化学对 AuNPs 吸附的影响。将单克隆抗辣根过氧化物酶 IgG 抗体(抗 HRP)与丙烯酸 N-琥珀酰亚胺酯 (NSA) 或还原的二硫代双琥珀酰亚胺丙酸酯 (DSP) 反应以修饰赖氨酸残基。Zeta 电位测量证实,两种化学修饰都减少了蛋白质表面正电荷的局部区域,而 DSP 修饰结合了额外的游离硫醇。动态光散射证实天然和化学修饰的抗体吸附在 AuNP 上形成生物偶联物;然而,吸附动力学表明,NSA 修饰的抗体需要更多的时间来形成硬电晕。此外,与 NSA 修饰的抗体形成的结合物失去了抗原结合功能,而未修饰和 DSP 修饰的抗体吸附在 AuNP 上形成功能性结合物。这些结果表明,当吸附在 AuNP 表面时,需要高亲和力官能团来防止蛋白质解折叠和功能丧失。DSP 修饰抗体上减少的蛋白质电荷和高亲和力硫醇基团能够实现蛋白质方向的 pH 依赖性控制,并在溶液 pH 值 (<7. 5) 由于偶联物聚集,未经修饰的抗体无法获得。该研究建立了蛋白质修饰参数,以促进高功能和稳定的蛋白质-AuNP 偶联物的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号