当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Simple 1–1 Electrolyte: Volumetric Properties of Aqueous Solutions of Sulfuric Acid at Elevated Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-07 , DOI: 10.1021/acs.jced.1c00291 Tuomas Vielma 1 , Lubomir Hnedkovsky 2 , Glenn Hefter 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-07 , DOI: 10.1021/acs.jced.1c00291 Tuomas Vielma 1 , Lubomir Hnedkovsky 2 , Glenn Hefter 2
Affiliation
|
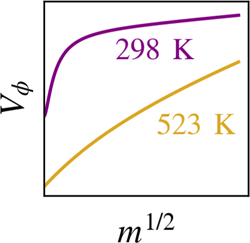
Densities of aqueous solutions of sulfuric acid (H2SO4) have been measured by vibrating tube densimetry at molalities m varying from 0.02 to 3.0 mol·kg–1, at temperatures over the range 323.15 ≤ T/K ≤ 523.15 at 10 MPa pressure. These results were used to calculate the corresponding apparent molar volumes Vϕ(H2SO4,aq) and appear to be the first-ever systematic study of the volumetric properties of this important acid at elevated temperatures. At T ≤ 373.15 K, the present results aligned well with recent literature data and with values reported in the 1926 International Critical Tables. No reliable experimental data were found for comparison at higher temperatures. The present results confirm that at lower concentrations and temperatures, sulfuric acid solutions exist as a variable mixture of H+(aq) + HSO4–(aq) + SO42–(aq). However, at higher temperatures, the degree of association increases markedly such that at T ≥ 448.15 K, sulfuric acid solutions behave like a simple 1:1 electrolyte [H+(aq) + HSO4–(aq)] even at low concentrations. The variation of Vϕ(H2SO4,aq) over the entire experimental region was well modelled using a simple Pitzer equation that specifically included (where appropriate) the effects of chemical speciation. Combination of this model with relevant literature data enabled estimation of the standard ionic volume V°(HSO4–,aq) and the standard volume change ΔrV° for the reaction H+(aq) + SO42–(aq) → HSO4–(aq) at temperatures up to 523 K.
中文翻译:

一种简单的 1-1 电解质:高温下硫酸水溶液的体积特性
硫酸 (H 2 SO 4 )水溶液的密度已通过振动管密度计测量,摩尔浓度m为 0.02 至 3.0 mol·kg –1,温度范围为 323.15 ≤ T /K ≤ 523.15,压力为 10 MPa . 这些结果用于计算相应的表观摩尔体积V ϕ (H 2 SO 4 ,aq),并且似乎是对这种重要酸在高温下的体积特性的首次系统研究。在T ≤ 373.15 K 时,目前的结果与最近的文献数据和 1926国际关键表。没有找到可靠的实验数据用于在较高温度下进行比较。目前的结果证实,在较低浓度和温度下,硫酸溶液以 H + (aq) + HSO 4 – (aq) + SO 4 2– (aq)的可变混合物形式存在。然而,在较高温度下,缔合程度显着增加,以至于在T ≥ 448.15 K 时,即使在低浓度下,硫酸溶液也表现得像一个简单的 1:1 电解质 [H + (aq) + HSO 4 – (aq)]。的变化V φ(H 2 SO 4,aq) 在整个实验区域内使用简单的 Pitzer 方程很好地建模,该方程特别包括(在适当的情况下)化学物种形成的影响。将该模型与相关文献数据相结合,可以估计标准离子体积V °(HSO 4 – ,aq) 和反应 H + (aq) + SO 4 2– (aq)的标准体积变化 Δ r V ° → HSO 4 – (aq) 在高达 523 K 的温度下。
更新日期:2021-08-12
中文翻译:

一种简单的 1-1 电解质:高温下硫酸水溶液的体积特性
硫酸 (H 2 SO 4 )水溶液的密度已通过振动管密度计测量,摩尔浓度m为 0.02 至 3.0 mol·kg –1,温度范围为 323.15 ≤ T /K ≤ 523.15,压力为 10 MPa . 这些结果用于计算相应的表观摩尔体积V ϕ (H 2 SO 4 ,aq),并且似乎是对这种重要酸在高温下的体积特性的首次系统研究。在T ≤ 373.15 K 时,目前的结果与最近的文献数据和 1926国际关键表。没有找到可靠的实验数据用于在较高温度下进行比较。目前的结果证实,在较低浓度和温度下,硫酸溶液以 H + (aq) + HSO 4 – (aq) + SO 4 2– (aq)的可变混合物形式存在。然而,在较高温度下,缔合程度显着增加,以至于在T ≥ 448.15 K 时,即使在低浓度下,硫酸溶液也表现得像一个简单的 1:1 电解质 [H + (aq) + HSO 4 – (aq)]。的变化V φ(H 2 SO 4,aq) 在整个实验区域内使用简单的 Pitzer 方程很好地建模,该方程特别包括(在适当的情况下)化学物种形成的影响。将该模型与相关文献数据相结合,可以估计标准离子体积V °(HSO 4 – ,aq) 和反应 H + (aq) + SO 4 2– (aq)的标准体积变化 Δ r V ° → HSO 4 – (aq) 在高达 523 K 的温度下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号