Journal of Luminescence ( IF 3.6 ) Pub Date : 2021-07-06 , DOI: 10.1016/j.jlumin.2021.118320 Cheng Fu 1 , Dongdong Zhang 1 , Gongnv Xu 1 , Xuankai Deng 2 , Min Liu 1 , Yun Deng 1 , Wangting Lu 1 , Chaohui Chen 1 , Yibin Ruan 3 , Yanhua Yu 1
|
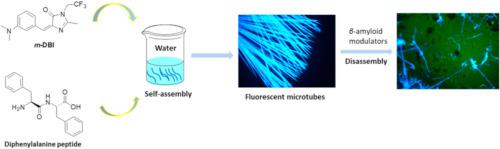
It is known that misfolding and aggregation of amyloid-beta (Aβ) peptide was thought to be the causing of Alzheimer's disease (AD). Until now, there are no effective drugs for this disease. Therefore, it is necessary to develop excellent probes for early diagnosis and assisting drug research and development of AD. Diphenylalanine peptide (FF), as a key and the smallest core recognition sequence of amyloid-beta (Aβ) peptide, has attracted much attention. In this paper, we designed and synthesized meta- and para-amino green fluorescent protein chromophore analogues m-DBI and p-DBI. Comparing their fluorescence properties, we found that m-DBI displayed relatively strong fluorescence in aprotic organic solvents due to a large barrier for the Z→E photoisomerization, while it showed weak fluorescence in protic solvents as a result of solvent-solute H-bonding interactions. Importantly, it could drive and assemble with FF into microtubes with “turn-on” fluorescence. In contrast, p-DBI exhibited very weak fluorescence in both protic and aprotic solvents, which is due to the ultrafast Z→E photoisomerization. Furthermore, it could not co-assemble with FF and even displayed weak fluorescence in solid state. In addition, FF-m-DBI co-assembly microtubes could be partially disassembled through incubating with a potent inhibitor of Aβ aggregation EGCG, accompanied by a decrease in fluorescence intensity, shows the potential application of screening possible inhibitor molecules of AD diseases.
中文翻译:

一种促进二苯丙氨酸自组装成荧光微管的绿色荧光蛋白发色团类似物
众所周知,淀粉样蛋白-β (Aβ) 肽的错误折叠和聚集被认为是阿尔茨海默病 (AD) 的原因。到目前为止,还没有针对这种疾病的有效药物。因此,有必要开发用于AD早期诊断和辅助药物研发的优良探针。二苯丙氨酸肽(FF)作为β-淀粉样蛋白(Aβ)肽的关键和最小的核心识别序列,备受关注。在本文中,我们设计并合成了间氨基和对氨基绿色荧光蛋白发色团类似物m -DBI 和p -DBI。比较它们的荧光特性,我们发现m-DBI 在非质子有机溶剂中由于 Z→E 光异构化的障碍很大,显示出相对强的荧光,而在质子溶剂中由于溶剂 - 溶质 H 键相互作用,它显示出较弱的荧光。重要的是,它可以驱动 FF 并将其组装成具有“开启”荧光的微管。相比之下,p- DBI 在质子和非质子溶剂中都表现出非常弱的荧光,这是由于超快的 Z→E 光异构化。此外,它不能与 FF 共组装,甚至在固态下显示出微弱的荧光。此外,FF- m-DBI 共组装微管可以通过与 Aβ 聚集的强抑制剂 EGCG 一起孵育而部分分解,伴随着荧光强度的降低,显示了筛选 AD 疾病可能的抑制剂分子的潜在应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号