当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Densities and Viscosities of Binary and Ternary Solutions of Triethylenetetramine, 2-Amino-2-methyl-1-propanol, and Water for Carbon Dioxide Capture
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-05 , DOI: 10.1021/acs.jced.0c01052 Atefeh Rezaei 1 , Peyman Pakzad 1 , Masoud Mofarahi 1, 2 , Amir Abbas Izadpanah 1 , Morteza Afkhamipour 1 , Chang-Ha Lee 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-05 , DOI: 10.1021/acs.jced.0c01052 Atefeh Rezaei 1 , Peyman Pakzad 1 , Masoud Mofarahi 1, 2 , Amir Abbas Izadpanah 1 , Morteza Afkhamipour 1 , Chang-Ha Lee 2
Affiliation
|
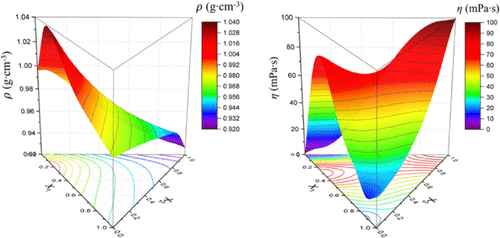
The densities and viscosities of three solutions, namely, two binary solutions [triethylenetetramine (TETA) + H2O and TETA + 2-amino-2-methyl-1-propanol (AMP)] and one ternary solution (TETA + AMP + H2O) were measured at atmospheric pressure and in the temperature range of 303.15 to 343.15 K. Their viscometric and volumetric characteristics, such as the excess molar volume (VE), partial molar volume (V̅i), excess partial molar volume (V̅iE), partial molar volume at infinite dilution (V̅i∞), excess partial molar volume at infinite dilution (V̅iE,∞), apparent molar volume (Vφ.i), viscosity deviation (Δη), molar Gibbs free energy (ΔG), molar entropy (ΔS), and molar enthalpy (ΔH), were calculated to determine the viscous flows of the solutions, and they could also explain the empirical results theoretically. The degrees of the decrease in viscosities and densities of the solutions were different. The variations in the properties were also analyzed through their intermolecular interactions and molecular structures. Additionally, Δη and VE were obtained by the Redlich–Kister model, for the binary solutions, and the Cibulka model, for the ternary solution. With the given standard deviations, σ ≤ 3.55, for the binary solutions, and σ = 3.98, for the ternary solution, the viscosity data were parameterized by the Eyring–non-random two-liquid model. The obtained thermodynamic properties were reliable and could be employed in the design and optimization of a CO2-capture process, utilizing the studied solutions.
中文翻译:

用于二氧化碳捕获的三亚乙基四胺、2-氨基-2-甲基-1-丙醇和水的二元和三元溶液的密度和粘度
三种溶液的密度和粘度,即两种二元溶液 [三亚乙基四胺 (TETA) + H 2 O 和 TETA + 2-氨基-2-甲基-1-丙醇 (AMP)] 和一种三元溶液 (TETA + AMP + H 2 O) 在大气压和 303.15 至 343.15 K 的温度范围内测量。它们的粘度和体积特性,例如过量摩尔体积 ( V E )、偏摩尔体积 ( V̅ i )、过量偏摩尔体积 ( V̅ i E ), 无限稀释时的偏摩尔体积 ( V̅ i ∞ ), 无限稀释时的过量偏摩尔体积 ( V̅ i E, ∞)、表观摩尔体积 ( V φ. i )、粘度偏差 (Δn)、摩尔吉布斯自由能 (Δ G )、摩尔熵 (Δ S ) 和摩尔焓 (Δ H ) 被计算以确定解,它们也可以从理论上解释经验结果。溶液的粘度和密度降低的程度不同。还通过它们的分子间相互作用和分子结构分析了性质的变化。此外,Δη 和V E由 Redlich-Kister 模型获得,对于二元解,以及 Cibulka 模型,对于三元解。对于给定的标准偏差,对于二元溶液,σ ≤ 3.55,对于三元溶液,σ = 3.98,粘度数据由 Eyring-非随机两液体模型参数化。获得的热力学性质是可靠的,可用于设计和优化 CO 2捕获过程,利用研究的解决方案。
更新日期:2021-08-12
中文翻译:

用于二氧化碳捕获的三亚乙基四胺、2-氨基-2-甲基-1-丙醇和水的二元和三元溶液的密度和粘度
三种溶液的密度和粘度,即两种二元溶液 [三亚乙基四胺 (TETA) + H 2 O 和 TETA + 2-氨基-2-甲基-1-丙醇 (AMP)] 和一种三元溶液 (TETA + AMP + H 2 O) 在大气压和 303.15 至 343.15 K 的温度范围内测量。它们的粘度和体积特性,例如过量摩尔体积 ( V E )、偏摩尔体积 ( V̅ i )、过量偏摩尔体积 ( V̅ i E ), 无限稀释时的偏摩尔体积 ( V̅ i ∞ ), 无限稀释时的过量偏摩尔体积 ( V̅ i E, ∞)、表观摩尔体积 ( V φ. i )、粘度偏差 (Δn)、摩尔吉布斯自由能 (Δ G )、摩尔熵 (Δ S ) 和摩尔焓 (Δ H ) 被计算以确定解,它们也可以从理论上解释经验结果。溶液的粘度和密度降低的程度不同。还通过它们的分子间相互作用和分子结构分析了性质的变化。此外,Δη 和V E由 Redlich-Kister 模型获得,对于二元解,以及 Cibulka 模型,对于三元解。对于给定的标准偏差,对于二元溶液,σ ≤ 3.55,对于三元溶液,σ = 3.98,粘度数据由 Eyring-非随机两液体模型参数化。获得的热力学性质是可靠的,可用于设计和优化 CO 2捕获过程,利用研究的解决方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号