Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-07-06 , DOI: 10.1016/j.atmosenv.2021.118603 Zhier Bao 1 , Huifeng Xu 1 , Kangwei Li 2 , Linghong Chen 1 , Xin Zhang 1 , Xuecheng Wu 1 , Xiang Gao 1 , Merched Azzi 3 , Kefa Cen 1
|
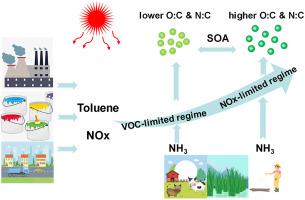
Ammonia (NH3) is an important base gas in the atmosphere and has received extensive research interest due to its role on secondary aerosol formation. However, the effects of NH3 on the photochemical smog oxidants formation including ozone and secondary aerosol did not attract much attention. In this study, a set of smog chamber experiments was conducted under toluene/NOx photo-oxidation base condition and adding NH3 in the following photochemical regimes referred as hydrocarbon, NOx-limited and transitional regimes. Dedicated instruments including gas analysers, scanning mobility particle sizer (SMPS), aerosol mass spectrometry (HR-ToF-AMS) were used to measure the gaseous compounds, aerosol mass concentration and chemical composition. It was found that the presence of NH3 did not affect the O3 formation rate. However, the particle number concentration increased dramatically after NH3 was injected in any photochemical regimes. This effect has been mainly attributed to the rapid formation of organic ammonium and ammonium nitrate. The mass spectra of organic aerosols from AMS showed that the most abundant fragments were at m/z 28, 29, 43 and 44, which became higher after the addition of NH3 in the transition and NOx-limited regimes, indicating that NH3 could enhance the formation of the compounds containing carbonyl and carboxylic acid functional groups. Compared to hydrocarbon-limited regime, higher atomic nitrogen-to-carbon (N:C) ratios were observed when NH3 were injected into transition and NOx-limited regimes, and this is mainly due to more organic nitrate formation. Four factors, which were assigned to inorganic nitrate aerosols, carboxylic acid compounds, carbonyl compounds and N-containing organic compounds (NOC), were identified and interpreted by applying the positive matrix factorization (PMF) to the AMS dataset. These results provide new insights into the complex chemistry of toluene/NOx/NH3 during different O3 formation regimes, and imply the benefit of controlling NH3 to mitigate PM2.5 when O3 formation is under VOC-limited regime.
中文翻译:

NH 3对不同O 3形成机制下甲苯/NOx光氧化二次气溶胶形成的影响
氨 (NH 3 ) 是大气中一种重要的基础气体,由于其在二次气溶胶形成中的作用而受到广泛的研究兴趣。然而,NH 3对包括臭氧和二次气溶胶在内的光化学烟雾氧化剂形成的影响并未引起太多关注。本研究在甲苯/NOx光氧化基条件下,加入NH 3进行了一组烟雾室实验。在以下称为碳氢化合物、NOx 限制和过渡状态的光化学状态中。使用气体分析仪、扫描迁移率粒度仪 (SMPS)、气溶胶质谱 (HR-ToF-AMS) 等专用仪器测量气态化合物、气溶胶质量浓度和化学成分。发现NH 3的存在不影响O 3形成速率。然而,NH 3后粒子数浓度急剧增加以任何光化学方式注入。这种效应主要归因于有机铵和硝酸铵的快速形成。来自 AMS 的有机气溶胶的质谱显示,最丰富的碎片位于 m/z 28、29、43 和 44,在过渡和 NOx 限制区域中加入 NH 3后,这些碎片变得更高,表明 NH 3可以促进含有羰基和羧酸官能团的化合物的形成。与碳氢化合物限制体系相比,当 NH 3被注入过渡和 NOx 限制状态,这主要是由于更多的有机硝酸盐形成。通过将正矩阵分解 (PMF) 应用于 AMS 数据集,识别和解释了四个因素,这些因素分配给无机硝酸盐气溶胶、羧酸化合物、羰基化合物和含氮有机化合物 (NOC)。这些结果提供了对不同 O 3形成过程中甲苯/NOx/NH 3复杂化学的新见解,并暗示了当 O 3形成处于 VOC 限制状态时控制 NH 3以减轻 PM 2.5的好处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号