当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the solvent on the Lewis acidity of antimony pentahalides and group 13 Lewis acids toward acetonitrile and pyridine
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2021-07-05 , DOI: 10.1002/jcc.26713 Anna V Pomogaeva 1 , Alexey Y Timoshkin 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2021-07-05 , DOI: 10.1002/jcc.26713 Anna V Pomogaeva 1 , Alexey Y Timoshkin 1
Affiliation
|
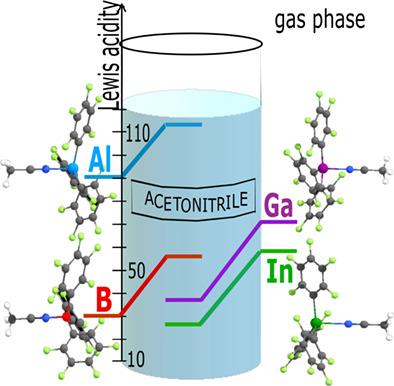
Energetic effects of solvation of SbF5, SbCl5, and 21 group 13 Lewis acids (LA) and their molecular complexes with acetonitrile and pyridine are evaluated using SMD approach. Compared to the gas phase, solvation increases the stability of boron- and aluminum-containing complexes but decreases the stability of gallium and indium-containing homologs due to larger solation energies of free LA. New Lewis acidity scales, based on the Gibbs energy of dissociation of the molecular complexes LA·pyridine and LA·acetonitrile in the gas phase, in benzene and acetonitrile solutions, are proposed.
中文翻译:

溶剂对五卤化锑和第 13 族路易斯酸对乙腈和吡啶的路易斯酸度的影响
使用 SMD 方法评估SbF 5、SbCl 5和 21 族 13 路易斯酸 (LA) 及其与乙腈和吡啶的分子复合物的溶剂化的能量效应。与气相相比,溶剂化提高了含硼和铝配合物的稳定性,但由于游离 LA 的溶化能较大,因此降低了含镓和铟同系物的稳定性。提出了新的路易斯酸度标度,基于分子复合物 LA·吡啶和 LA·乙腈在气相中、苯和乙腈溶液中的 Gibbs 离解能。
更新日期:2021-08-07
中文翻译:

溶剂对五卤化锑和第 13 族路易斯酸对乙腈和吡啶的路易斯酸度的影响
使用 SMD 方法评估SbF 5、SbCl 5和 21 族 13 路易斯酸 (LA) 及其与乙腈和吡啶的分子复合物的溶剂化的能量效应。与气相相比,溶剂化提高了含硼和铝配合物的稳定性,但由于游离 LA 的溶化能较大,因此降低了含镓和铟同系物的稳定性。提出了新的路易斯酸度标度,基于分子复合物 LA·吡啶和 LA·乙腈在气相中、苯和乙腈溶液中的 Gibbs 离解能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号