当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Compactness in Hen Egg White Lysozyme Induced by Bisphenol S: A Spectroscopic and Molecular Dynamics Simulation Approach
ChemPhysChem ( IF 2.3 ) Pub Date : 2021-07-05 , DOI: 10.1002/cphc.202100272 Ushasi Pramanik 1 , Anju Ajayan Kongasseri 1 , Shashi Shekhar 1 , Ashwin Mathew 1 , Rahul Yadav 1 , Saptarshi Mukherjee 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2021-07-05 , DOI: 10.1002/cphc.202100272 Ushasi Pramanik 1 , Anju Ajayan Kongasseri 1 , Shashi Shekhar 1 , Ashwin Mathew 1 , Rahul Yadav 1 , Saptarshi Mukherjee 1
Affiliation
|
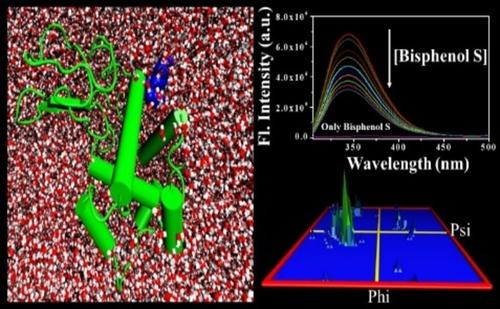
The endocrine disrupting compound Bisphenol and its analogues are widely used in food packaging products and can cause serious health hazards. The protein, Lysozyme (Lyz), showing anti-microbial properties, is used as a “natural” food and dairy preservative. Herein, we explored the interaction between Lyz and Bisphenol S (BPS) by multi-spectroscopic and theoretical approaches. Lyz interacts with BPS through static quenching, where hydrophobic force governed the underlying interaction. Molecular docking results reveal that tryptophan plays a vital role in binding, corroborated well with near UV-CD studies. A decrease in the radius of gyration (from 1.43 nm to 1.35 nm) of Lyz substantiates the compactness of the protein conformation owing to such an interaction. This structural alteration experienced by Lyz may alter its functional properties as a food preservative. Consequently, this can degrade the quality of the food products and thereby lead to severe health issues.
中文翻译:

双酚S诱导的鸡蛋清溶菌酶的结构致密性:光谱和分子动力学模拟方法
内分泌干扰化合物双酚及其类似物广泛用于食品包装产品,可对健康造成严重危害。蛋白质溶菌酶 (Lyz) 具有抗微生物特性,可用作“天然”食品和乳制品防腐剂。在此,我们通过多光谱和理论方法探索了 Lyz 和双酚 S (BPS) 之间的相互作用。Lyz 通过静态淬灭与 BPS 相互作用,其中疏水力控制了潜在的相互作用。分子对接结果表明,色氨酸在结合中起着至关重要的作用,这在近 UV-CD 研究中得到了很好的证实。由于这种相互作用,Lyz 的回转半径(从 1.43 nm 到 1.35 nm)的减小证实了蛋白质构象的紧凑性。Lyz 经历的这种结构改变可能会改变其作为食品防腐剂的功能特性。因此,这会降低食品的质量,从而导致严重的健康问题。
更新日期:2021-09-03
中文翻译:

双酚S诱导的鸡蛋清溶菌酶的结构致密性:光谱和分子动力学模拟方法
内分泌干扰化合物双酚及其类似物广泛用于食品包装产品,可对健康造成严重危害。蛋白质溶菌酶 (Lyz) 具有抗微生物特性,可用作“天然”食品和乳制品防腐剂。在此,我们通过多光谱和理论方法探索了 Lyz 和双酚 S (BPS) 之间的相互作用。Lyz 通过静态淬灭与 BPS 相互作用,其中疏水力控制了潜在的相互作用。分子对接结果表明,色氨酸在结合中起着至关重要的作用,这在近 UV-CD 研究中得到了很好的证实。由于这种相互作用,Lyz 的回转半径(从 1.43 nm 到 1.35 nm)的减小证实了蛋白质构象的紧凑性。Lyz 经历的这种结构改变可能会改变其作为食品防腐剂的功能特性。因此,这会降低食品的质量,从而导致严重的健康问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号