当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 4-Trifluoromethylated 1,3-Butadienes via Palladium Catalyzed Heck Reaction
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-07-05 , DOI: 10.1002/cjoc.202100313 Yang Li 1 , Meng Hao 1 , Yu‐Chen Chang 1 , Yuan Liu 1 , Wen‐Fei Wang 1 , Ning Sun 1 , Wen‐Qing Zhu 1 , Ziwei Gao 2, 3
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-07-05 , DOI: 10.1002/cjoc.202100313 Yang Li 1 , Meng Hao 1 , Yu‐Chen Chang 1 , Yuan Liu 1 , Wen‐Fei Wang 1 , Ning Sun 1 , Wen‐Qing Zhu 1 , Ziwei Gao 2, 3
Affiliation
|
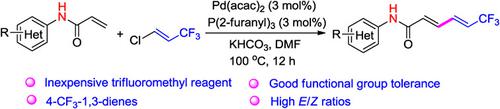
1,3-Butadiene plays a key role in modern synthetic chemistry and biochemistry because it is a key intermediate in the synthesis of many drugs. A new and effective method for the synthesis of 4-trifluoromethylated 1,3-butadiene through the fluorinated Heck reaction catalyzed by Pd(0) is described. Without additives, 1-chloro-3,3,3-trifluoropropene (an inexpensive CF3 structural unit that is harmless to ozone) reacts with enamide to synthesize 4-trifluoromethylated 1,3-butadienes with good yield, high regioselectivity and chemical selectivity, and strong tolerance of substrate functional groups such as alkynes, aldehyde, and ester groups.
中文翻译:

钯催化 Heck 反应合成 4-三氟甲基化 1,3-丁二烯
1,3-丁二烯在现代合成化学和生物化学中起着关键作用,因为它是许多药物合成的关键中间体。描述了一种通过 Pd(0) 催化的氟化 Heck 反应合成 4-三氟甲基化 1,3-丁二烯的新方法。在没有添加剂的情况下,1-氯-3,3,3-三氟丙烯(一种对臭氧无害的廉价CF 3结构单元)与烯酰胺反应合成4-三氟甲基化1,3-丁二烯,具有良好的收率、高区域选择性和化学选择性,对炔、醛、酯基等底物官能团的耐受性强。
更新日期:2021-07-05

中文翻译:

钯催化 Heck 反应合成 4-三氟甲基化 1,3-丁二烯
1,3-丁二烯在现代合成化学和生物化学中起着关键作用,因为它是许多药物合成的关键中间体。描述了一种通过 Pd(0) 催化的氟化 Heck 反应合成 4-三氟甲基化 1,3-丁二烯的新方法。在没有添加剂的情况下,1-氯-3,3,3-三氟丙烯(一种对臭氧无害的廉价CF 3结构单元)与烯酰胺反应合成4-三氟甲基化1,3-丁二烯,具有良好的收率、高区域选择性和化学选择性,对炔、醛、酯基等底物官能团的耐受性强。












































 京公网安备 11010802027423号
京公网安备 11010802027423号