Archives of Biochemistry and Biophysics ( IF 3.9 ) Pub Date : 2021-07-03 , DOI: 10.1016/j.abb.2021.108980 Sasiprapa Samsri 1 , Soraya Pornsuwan 1
|
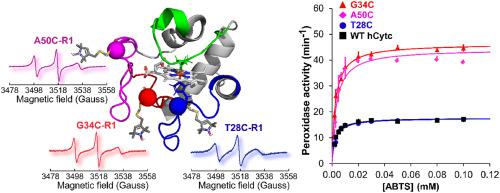
Cytochrome c (Cytc) is a multifunctional protein associated with electron shuttling in the inner membrane of mitochondria and also involving in the apoptotic pathway. It has been identified that mutations located in the flexible central 40-57 Ω-loop including the naturally occurring G41S, Y48H, and A51V mutants, which are found in patients with thrombocytopenia 4, a platelet disorder, alter the structural properties of human Cytc (hCytc) that associated to enhanced peroxidase activity. In this work we compared the cysteine-directed mutants of hCytc located in three different parts of Ω-loops, i.e., T28C and G34C (proximal Ω-loop), and A50C (central Ω-loop), with respect to the wild-type (WT) hCytc. The mutants and WT hCytc were structurally characterized by circular dichroism, heating and chemical denaturations, and fluorescence spectroscopy. The flexibility at the cysteine mutated sites was directly determined by site-directed spin-labeling Electron Spin Resonance. Alkaline transitions were determined by pH titration and the alkaline conformers were related to peroxidase activity of all hCytc proteins. Structural and dynamic characterizations were rationally correlated to the modulation of peroxidase activity in these mutants in comparison to the WT hCytc. We found that the cysteine mutations at residues T28 and G34, both located in the same region of Ω-loop, developed different conformations and dynamical properties that lead to different effects on the rates of peroxidase activity (G34C was ~2.6 folds higher), whereas the rate of G34C was closer to that of A50C mutant. The results implied that the flexibility and local structures of the proximal Ω-loop could also play an important role in modulating the peroxidase activity which can be associated to apoptosis.
中文翻译:

Ω环处半胱氨酸定向突变对人细胞色素c过氧化物酶活性的影响
细胞色素 c (Cytc) 是一种多功能蛋白质,与线粒体内膜中的电子穿梭相关,也参与细胞凋亡途径。已经确定位于灵活的中央 40-57 Ω 环中的突变包括天然存在的 G41S、Y48H 和 A51V 突变体,这些突变体在血小板减少症 4(一种血小板疾病)患者中发现,改变了人类 Cytc 的结构特性。 hCytc) 与增强的过氧化物酶活性有关。在这项工作中,我们比较了位于 Ω 环三个不同部分的 hCytc 的半胱氨酸导向突变体,即 T28C 和 G34C(近端 Ω 环)和 A50C(中央 Ω 环),相对于野生型(WT) hCytc。突变体和 WT hCytc 的结构特征在于圆二色性、加热和化学变性以及荧光光谱。半胱氨酸突变位点的灵活性由定点自旋标记电子自旋共振直接确定。碱性转变由 pH 滴定确定,碱性构象异构体与所有 hCytc 蛋白的过氧化物酶活性有关。与 WT hCytc 相比,结构和动态特征与这些突变体中过氧化物酶活性的调节合理相关。我们发现残基 T28 和 G34 处的半胱氨酸突变均位于 Ω 环的同一区域,形成了不同的构象和动力学特性,导致对过氧化物酶活性速率的不同影响(G34C 高约 2.6 倍),而G34C 的比率更接近 A50C 突变体的比率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号