当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Equilibrium and Binodal Curves for Aqueous Solutions of NH3, NH4HCO3, MDEA, and K2CO3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-02 , DOI: 10.1021/acs.jced.1c00144 Lucas Farias Falcchi Corrêa 1 , Kaj Thomsen 1 , Philip Loldrup Fosbøl 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-02 , DOI: 10.1021/acs.jced.1c00144 Lucas Farias Falcchi Corrêa 1 , Kaj Thomsen 1 , Philip Loldrup Fosbøl 1
Affiliation
|
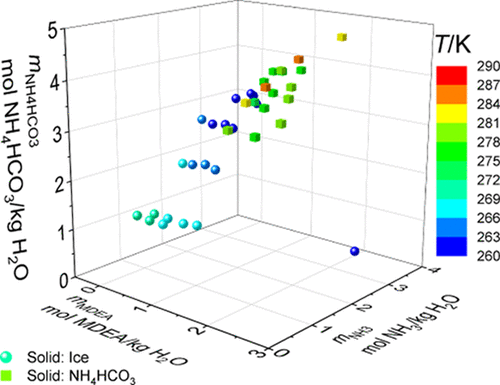
Solid–liquid equilibrium (SLE) data were obtained at 0.1 MPa for binary, ternary, and quaternary aqueous solutions containing ammonia (NH3), ammonium bicarbonate (NH4HCO3), methyl diethanolamine (MDEA), and potassium carbonate (K2CO3) using a modified Beckmann apparatus. The reproducibility of the experimental method was verified by measuring the SLE of aqueous solutions containing NH3, K2CO3, or MDEA in a temperature range between 237 and 273 K. A total of 120 new data points were measured for MDEA–NH3 and NH4HCO3–H2O mixtures (250 to 305 K), and 23 new data points were obtained for MDEA–K2CO3–H2O solutions (250 to 270 K). These measurements allow for an accurate estimation of water activity in such mixtures and provide insights on the limits of solid formation. The results indicate that the addition of MDEA increases the solubility of NH4HCO3, whereas a liquid–liquid split was observed when K2CO3 was added to aqueous MDEA. This opens the possibility of using it as a phase demixing solvent for carbon capture. Despite the extensive literature regarding mixed salt–amine solutions, liquid demixing in such systems has not been reported before. For this reason, the binodal curve for the MDEA–K2CO3–H2O solution was measured at 293.15 K using the method of cloud point titration. These results assist the selection of operation parameters that avoid a liquid–liquid split in the process.
中文翻译:

NH3、NH4HCO3、MDEA 和 K2CO3 水溶液的固液平衡和双节曲线
含氨 (NH 3 )、碳酸氢铵 (NH 4 HCO 3 )、甲基二乙醇胺 (MDEA) 和碳酸钾 (K 2 CO 3 ) 使用改进的贝克曼装置。通过在 237 到 273 K 的温度范围内测量含有 NH 3、K 2 CO 3或 MDEA的水溶液的 SLE 来验证实验方法的重现性。共测量了 120 个 MDEA–NH 3 的新数据点和 NH 4 HCO 3 –H 2O 混合物(250 至 305 K),并获得了 MDEA–K 2 CO 3 –H 2 O 溶液(250 至 270 K)的23 个新数据点。这些测量允许准确估计此类混合物中的水活度,并提供有关固体形成限制的见解。结果表明,加入 MDEA 增加了 NH 4 HCO 3的溶解度,而当 K 2 CO 3加入MDEA水溶液中。这开启了将其用作碳捕获的相分离溶剂的可能性。尽管有大量关于盐-胺混合溶液的文献,但以前从未报道过此类系统中的液体分层。出于这个原因,MDEA–K 2 CO 3 –H 2 O 溶液的双峰曲线是在 293.15 K 下使用浊点滴定法测量的。这些结果有助于选择操作参数,以避免在过程中发生液-液分裂。
更新日期:2021-08-12
中文翻译:

NH3、NH4HCO3、MDEA 和 K2CO3 水溶液的固液平衡和双节曲线
含氨 (NH 3 )、碳酸氢铵 (NH 4 HCO 3 )、甲基二乙醇胺 (MDEA) 和碳酸钾 (K 2 CO 3 ) 使用改进的贝克曼装置。通过在 237 到 273 K 的温度范围内测量含有 NH 3、K 2 CO 3或 MDEA的水溶液的 SLE 来验证实验方法的重现性。共测量了 120 个 MDEA–NH 3 的新数据点和 NH 4 HCO 3 –H 2O 混合物(250 至 305 K),并获得了 MDEA–K 2 CO 3 –H 2 O 溶液(250 至 270 K)的23 个新数据点。这些测量允许准确估计此类混合物中的水活度,并提供有关固体形成限制的见解。结果表明,加入 MDEA 增加了 NH 4 HCO 3的溶解度,而当 K 2 CO 3加入MDEA水溶液中。这开启了将其用作碳捕获的相分离溶剂的可能性。尽管有大量关于盐-胺混合溶液的文献,但以前从未报道过此类系统中的液体分层。出于这个原因,MDEA–K 2 CO 3 –H 2 O 溶液的双峰曲线是在 293.15 K 下使用浊点滴定法测量的。这些结果有助于选择操作参数,以避免在过程中发生液-液分裂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号