Cell ( IF 45.5 ) Pub Date : 2021-07-02 , DOI: 10.1016/j.cell.2021.05.045 Allon Wagner 1 , Chao Wang 2 , Johannes Fessler 3 , David DeTomaso 4 , Julian Avila-Pacheco 5 , James Kaminski 4 , Sarah Zaghouani 3 , Elena Christian 5 , Pratiksha Thakore 5 , Brandon Schellhaass 6 , Elliot Akama-Garren 3 , Kerry Pierce 5 , Vasundhara Singh 5 , Noga Ron-Harel 7 , Vivian Paraskevi Douglas 8 , Lloyd Bod 3 , Alexandra Schnell 3 , Daniel Puleston 9 , Raymond A Sobel 10 , Marcia Haigis 11 , Erika L Pearce 9 , Manoocher Soleimani 12 , Clary Clish 5 , Aviv Regev 13 , Vijay K Kuchroo 14 , Nir Yosef 15
|
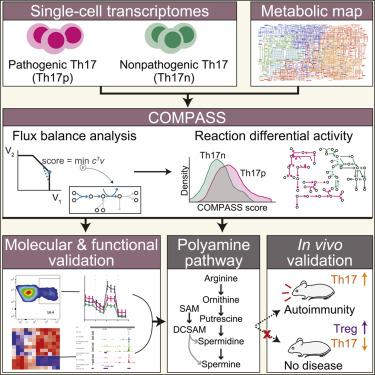
Metabolism is a major regulator of immune cell function, but it remains difficult to study the metabolic status of individual cells. Here, we present Compass, an algorithm to characterize cellular metabolic states based on single-cell RNA sequencing and flux balance analysis. We applied Compass to associate metabolic states with T helper 17 (Th17) functional variability (pathogenic potential) and recovered a metabolic switch between glycolysis and fatty acid oxidation, akin to known Th17/regulatory T cell (Treg) differences, which we validated by metabolic assays. Compass also predicted that Th17 pathogenicity was associated with arginine and downstream polyamine metabolism. Indeed, polyamine-related enzyme expression was enhanced in pathogenic Th17 and suppressed in Treg cells. Chemical and genetic perturbation of polyamine metabolism inhibited Th17 cytokines, promoted Foxp3 expression, and remodeled the transcriptome and epigenome of Th17 cells toward a Treg-like state. In vivo perturbations of the polyamine pathway altered the phenotype of encephalitogenic T cells and attenuated tissue inflammation in CNS autoimmunity.
中文翻译:

单个 Th17 细胞的代谢模型揭示了自身免疫的调节因子
代谢是免疫细胞功能的主要调节因子,但研究单个细胞的代谢状态仍然很困难。在这里,我们提出了 Compass,一种基于单细胞 RNA 测序和通量平衡分析来表征细胞代谢状态的算法。我们应用 Compass 将代谢状态与 T 辅助细胞 17 (Th17) 功能变异性(致病潜力)联系起来,并恢复了糖酵解和脂肪酸氧化之间的代谢转换,类似于已知的 Th17/调节性 T 细胞 (Treg) 差异,我们通过代谢验证了这一点化验。 Compass 还预测 Th17 致病性与精氨酸和下游多胺代谢有关。事实上,多胺相关酶的表达在致病性 Th17 细胞中增强,而在 Treg 细胞中表达受到抑制。多胺代谢的化学和遗传扰动抑制了 Th17 细胞因子,促进 Foxp3 表达,并将 Th17 细胞的转录组和表观基因组重塑为 Treg 样状态。体内多胺途径的扰动改变了致脑炎 T 细胞的表型,并减轻了中枢神经系统自身免疫中的组织炎症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号