Immunity ( IF 25.5 ) Pub Date : 2021-07-02 , DOI: 10.1016/j.immuni.2021.06.018 Anthony T DiPiazza 1 , Sarah R Leist 2 , Olubukola M Abiona 1 , Juan I Moliva 1 , Anne Werner 1 , Mahnaz Minai 3 , Bianca M Nagata 3 , Kevin W Bock 3 , Emily Phung 1 , Alexandra Schäfer 2 , Kenneth H Dinnon 2 , Lauren A Chang 1 , Rebecca J Loomis 1 , Seyhan Boyoglu-Barnum 1 , Gabriela S Alvarado 1 , Nancy J Sullivan 1 , Darin K Edwards 4 , Kaitlyn M Morabito 1 , John R Mascola 1 , Andrea Carfi 4 , Kizzmekia S Corbett 1 , Ian N Moore 3 , Ralph S Baric 2 , Barney S Graham 1 , Tracy J Ruckwardt 1
|
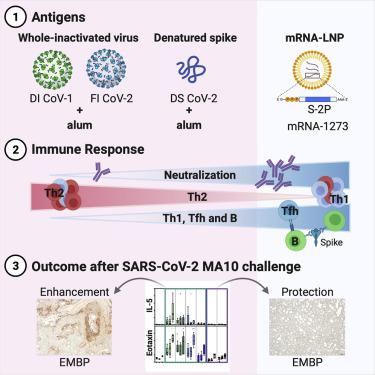
Vaccine-associated enhanced respiratory disease (VAERD) was previously observed in some preclinical models of severe acute respiratory syndrome (SARS) and MERS coronavirus vaccines. We used the SARS coronavirus 2 (SARS-CoV-2) mouse-adapted, passage 10, lethal challenge virus (MA10) mouse model of acute lung injury to evaluate the immune response and potential for immunopathology in animals vaccinated with research-grade mRNA-1273. Whole-inactivated virus or heat-denatured spike protein subunit vaccines with alum designed to elicit low-potency antibodies and Th2-skewed CD4+ T cells resulted in reduced viral titers and weight loss post challenge but more severe pathological changes in the lung compared to saline-immunized animals. In contrast, a protective dose of mRNA-1273 induced favorable humoral and cellular immune responses that protected from viral replication in the upper and lower respiratory tract upon challenge. A subprotective dose of mRNA-1273 reduced viral replication and limited histopathological manifestations compared to animals given saline. Overall, our findings demonstrate an immunological signature associated with antiviral protection without disease enhancement following vaccination with mRNA-1273.
中文翻译:

COVID-19 疫苗 mRNA-1273 在小鼠体内引发保护性免疫特征,该免疫特征与 SARS-CoV-2 攻击后疫苗增强的疾病无关
此前在严重急性呼吸综合征(SARS)和中东呼吸综合征(MERS)冠状病毒疫苗的一些临床前模型中观察到与疫苗相关的增强呼吸系统疾病(VAERD)。我们使用 SARS 冠状病毒 2 (SARS-CoV-2) 小鼠适应型第 10 代致死性攻击病毒 (MA10) 小鼠急性肺损伤模型来评估接种研究级 mRNA 疫苗的动物的免疫反应和免疫病理学潜力。 1273.与盐水相比,全灭活病毒或热变性刺突蛋白亚单位疫苗与明矾旨在引发低效抗体和 Th2 偏向 CD4 + T 细胞,导致攻击后病毒滴度降低和体重减轻,但肺部病理变化更严重- 已免疫的动物。相比之下,保护剂量的 mRNA-1273 诱导了良好的体液和细胞免疫反应,在受到攻击时可以保护上呼吸道和下呼吸道免受病毒复制。与给予盐水的动物相比,亚保护剂量的 mRNA-1273 减少了病毒复制并限制了组织病理学表现。总体而言,我们的研究结果证明了与抗病毒保护相关的免疫学特征,而在接种 mRNA-1273 后不会增强疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号