当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibria of H2S in Aqueous Mixtures of N-Methyldiethanolamine + Piperazine + Sulfolane
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-02 , DOI: 10.1021/acs.jced.1c00138 Mohammad Shokouhi 1 , Ali T. Zoghi 1, 2 , Amir H. Jalili 1, 3 , Ali Mehdizadeh 4
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-07-02 , DOI: 10.1021/acs.jced.1c00138 Mohammad Shokouhi 1 , Ali T. Zoghi 1, 2 , Amir H. Jalili 1, 3 , Ali Mehdizadeh 4
Affiliation
|
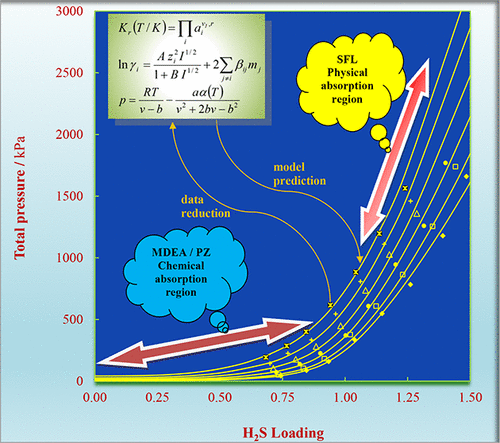
The solubility of hydrogen sulfide (H2S) in a mixed solvent containing (19.5 to 35.5) mass percent of N-methyldiethanolamine (MDEA), (0 to 11.6) mass percent of piperazine (PZ), (0 to 10) mass percent of sulfolane (SFL), and water at temperatures of (313.15 to 353.15) K and H2S partial pressures of up to about 2 MPa is reported. To determine the absorption of H2S, the density of the solutions in the corresponding temperature range was measured using a vibrating-tube densimeter. The results show that H2S solubility decreases with increasing temperature and increases with partial pressure of H2S. In addition, increasing PZ concentration can enhance the absorption capacity of MDEA, as a benchmark alkanolamine, in aqueous and mixed aqueous sulfolane solutions in the low-loading region. A thermodynamic model based on the extended Debye–Hükel theory, namely Deshmukh-Mather model for the liquid phase, combined with the Peng-Robinson equation of state for the gas phase, was applied to correlate the H2S solubility data obtained. The average absolute deviation of the correlated data from the experimental data is 6.5%, which implies good correlative accuracy of the thermodynamic model employed in this work.
中文翻译:

N-甲基二乙醇胺 + 哌嗪 + 环丁砜的水性混合物中 H2S 的气液平衡
硫化氢(H 2 S)在含有(19.5~35.5)质量百分比的N-甲基二乙醇胺(MDEA)、(0~11.6)质量百分比的哌嗪(PZ)、(0~10)质量百分比的混合溶剂中的溶解度据报道,环丁砜 (SFL) 和水在 (313.15 至 353.15) K 的温度和 H 2 S 分压高达约 2 兆帕。为了确定H 2 S的吸收,使用振动管密度计测量相应温度范围内溶液的密度。结果表明,H 2 S溶解度随温度升高而降低,随H 2分压升高而升高。S. 此外,增加 PZ 浓度可以增强 MDEA 作为基准烷醇胺,在低负载区的环丁砜水溶液和混合水溶液中的吸收能力。基于扩展的 Debye-Hükel 理论的热力学模型,即液相的 Deshmukh-Mather 模型,结合气相的 Peng-Robinson 状态方程,用于关联获得的 H 2 S 溶解度数据。相关数据与实验数据的平均绝对偏差为 6.5%,这意味着本工作中采用的热力学模型具有良好的相关精度。
更新日期:2021-07-02
中文翻译:

N-甲基二乙醇胺 + 哌嗪 + 环丁砜的水性混合物中 H2S 的气液平衡
硫化氢(H 2 S)在含有(19.5~35.5)质量百分比的N-甲基二乙醇胺(MDEA)、(0~11.6)质量百分比的哌嗪(PZ)、(0~10)质量百分比的混合溶剂中的溶解度据报道,环丁砜 (SFL) 和水在 (313.15 至 353.15) K 的温度和 H 2 S 分压高达约 2 兆帕。为了确定H 2 S的吸收,使用振动管密度计测量相应温度范围内溶液的密度。结果表明,H 2 S溶解度随温度升高而降低,随H 2分压升高而升高。S. 此外,增加 PZ 浓度可以增强 MDEA 作为基准烷醇胺,在低负载区的环丁砜水溶液和混合水溶液中的吸收能力。基于扩展的 Debye-Hükel 理论的热力学模型,即液相的 Deshmukh-Mather 模型,结合气相的 Peng-Robinson 状态方程,用于关联获得的 H 2 S 溶解度数据。相关数据与实验数据的平均绝对偏差为 6.5%,这意味着本工作中采用的热力学模型具有良好的相关精度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号