当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interleukin-35 is a critical regulator of immunity during helminth infections associated with multiple sclerosis
Immunology ( IF 4.9 ) Pub Date : 2021-07-01 , DOI: 10.1111/imm.13389 Jorge Correale 1 , Mariano Marrodan 1 , Edgar Carnero Contentti 2
Immunology ( IF 4.9 ) Pub Date : 2021-07-01 , DOI: 10.1111/imm.13389 Jorge Correale 1 , Mariano Marrodan 1 , Edgar Carnero Contentti 2
Affiliation
|
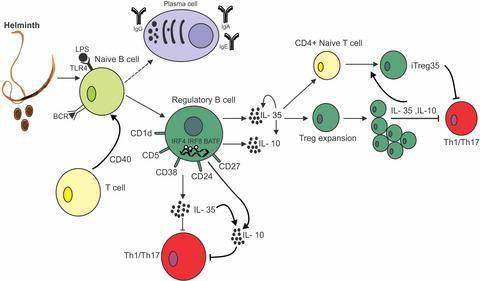
Multiple sclerosis (MS) is currently thought to arise by interactions between genetic susceptibility and environmental factors. Infections in general trigger autoimmune responses causing clinical manifestations of disease. However, as a result of regulatory T (Treg)- and regulatory B (Breg)-cell induction, helminth infections tend to dampen disease activity. IL-35, the newest member of the IL-12 family, is an inhibitory cytokine composed of an EBI3β chain subunit, and an IL-12p35 subunit. The aim of this study was to investigate the role of IL-35 during parasite infections occurring in individuals with MS. Numbers of IL-35-producing Breg cells are higher in CSF from helminth-infected than from uninfected MS subjects, a finding associated with decreased MRI disease activity. Interestingly, stimulation of CD19+ B cells with IL-35 promotes conversion of these cells to Breg cells producing both IL-35 and IL-10. Coculture of B cells from helminth-infected MS patients inhibits proliferation of Th1 and Th17 myelin peptide-specific T cells, as well as production of IFN-γ and IL-17. Following activation, CD4+CD25+ Treg cells significantly upregulate expression of EBI3 and IL-12p35 mRNA. Furthermore, CD4+CD25− T cells activated in the presence of IL-35 induce a population of cells with regulatory function, known as iTR35. Finally, B cells from normal individuals cultured in vitro in the presence of the helminth antigen SEA increase expression of the transcription BATF, IRF4 and IRF8, acquiring a pattern similar to that of IL-35 Breg cells. These data highlight the important immunoregulatory effects of IL-35 on both Breg and Treg cells, observed in helminth-infected MS subjects.
中文翻译:

白细胞介素 35 是与多发性硬化症相关的蠕虫感染期间免疫的关键调节剂
目前认为多发性硬化症 (MS) 是由遗传易感性和环境因素之间的相互作用引起的。感染通常会引发引起疾病临床表现的自身免疫反应。然而,由于调节性 T (Treg) 细胞和调节性 B (Breg) 细胞诱导,蠕虫感染往往会抑制疾病活动。IL-35是IL-12家族的最新成员,是由一个EBI3β链亚基和一个IL-12p35亚基组成的抑制性细胞因子。本研究的目的是调查 IL-35 在 MS 患者发生寄生虫感染过程中的作用。感染蠕虫的脑脊液中产生 IL-35 的 Breg 细胞数量高于未感染的 MS 受试者,这一发现与 MRI 疾病活动性降低有关。有趣的是,刺激 CD19 +具有 IL-35 的 B 细胞促进这些细胞转化为产生 IL-35 和 IL-10 的 Breg 细胞。来自蠕虫感染的 MS 患者的 B 细胞共培养可抑制 Th1 和 Th17 髓鞘肽特异性 T 细胞的增殖,以及 IFN-γ 和 IL-17 的产生。激活后,CD4 + CD25 + Treg 细胞显着上调EBI3和IL - 12p35 mRNA 的表达。此外,CD4 + CD25 -在 IL-35 存在下激活的 T 细胞会诱导具有调节功能的细胞群,称为 iTR35。最后,在蠕虫抗原 SEA 存在下体外培养的正常个体的 B 细胞增加了转录 BATF、IRF4 和 IRF8 的表达,获得了与 IL-35 Breg 细胞相似的模式。这些数据突出了 IL-35 对 Breg 和 Treg 细胞的重要免疫调节作用,在蠕虫感染的 MS 受试者中观察到。
更新日期:2021-07-01
中文翻译:

白细胞介素 35 是与多发性硬化症相关的蠕虫感染期间免疫的关键调节剂
目前认为多发性硬化症 (MS) 是由遗传易感性和环境因素之间的相互作用引起的。感染通常会引发引起疾病临床表现的自身免疫反应。然而,由于调节性 T (Treg) 细胞和调节性 B (Breg) 细胞诱导,蠕虫感染往往会抑制疾病活动。IL-35是IL-12家族的最新成员,是由一个EBI3β链亚基和一个IL-12p35亚基组成的抑制性细胞因子。本研究的目的是调查 IL-35 在 MS 患者发生寄生虫感染过程中的作用。感染蠕虫的脑脊液中产生 IL-35 的 Breg 细胞数量高于未感染的 MS 受试者,这一发现与 MRI 疾病活动性降低有关。有趣的是,刺激 CD19 +具有 IL-35 的 B 细胞促进这些细胞转化为产生 IL-35 和 IL-10 的 Breg 细胞。来自蠕虫感染的 MS 患者的 B 细胞共培养可抑制 Th1 和 Th17 髓鞘肽特异性 T 细胞的增殖,以及 IFN-γ 和 IL-17 的产生。激活后,CD4 + CD25 + Treg 细胞显着上调EBI3和IL - 12p35 mRNA 的表达。此外,CD4 + CD25 -在 IL-35 存在下激活的 T 细胞会诱导具有调节功能的细胞群,称为 iTR35。最后,在蠕虫抗原 SEA 存在下体外培养的正常个体的 B 细胞增加了转录 BATF、IRF4 和 IRF8 的表达,获得了与 IL-35 Breg 细胞相似的模式。这些数据突出了 IL-35 对 Breg 和 Treg 细胞的重要免疫调节作用,在蠕虫感染的 MS 受试者中观察到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号