当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
γ-Hydroxy Amides from Tartaric Acid: Versatile Chiral Building Blocks for the Total Synthesis of Natural Products
The Chemical Record ( IF 6.6 ) Pub Date : 2021-07-01 , DOI: 10.1002/tcr.202100129 Laxmi Narayan Nanda 1 , Kodambahalli S Shruthi 1 , Kavirayani R Prasad 1
The Chemical Record ( IF 6.6 ) Pub Date : 2021-07-01 , DOI: 10.1002/tcr.202100129 Laxmi Narayan Nanda 1 , Kodambahalli S Shruthi 1 , Kavirayani R Prasad 1
Affiliation
|
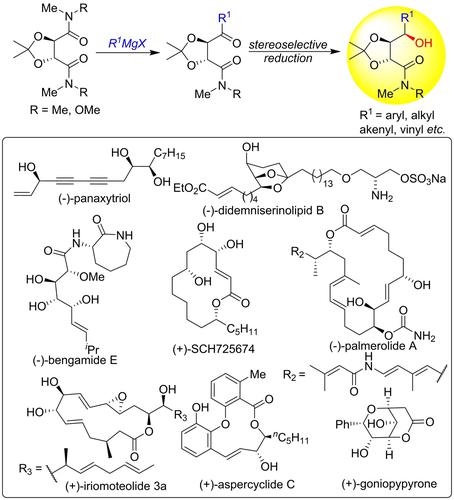
“Chiral pool” compounds possessing well defined stereocenters and suitable functionality serve as excellent building blocks for the synthesis of natural products and therapeutically important compounds. Tartaric acid is a C2-symmetric molecule available in both enantiomeric forms. It was extensively utilized in the synthesis of privileged chiral ligands/catalysts such as TADDOLs, and as a start point in the synthesis of plethora of compounds. The advent of several new C−C bond forming reactions offers opportunity for the development of novel synthetic strategies based on chiral pool compounds. We found that the desymmetrization of the bis-dimethyl amide/Weinreb amide derived from tartaric acid can be accomplished by controlled addition of Grignard /organolithium reagents leading to the mono keto amides, the reduction of which affords the γ-hydroxy amides. This account describes our research efforts of more than a decade on the synthesis and application of diverse γ-hydroxy amides derived from tartaric acid in the total synthesis of structurally simple to complex bio-active natural products.
中文翻译:

酒石酸中的 γ-羟基酰胺:用于天然产物全合成的多功能手性构件
“手性池”化合物具有明确定义的立体中心和合适的功能,是合成天然产物和具有治疗意义的化合物的极好基石。酒石酸是一种 C 2-对称分子有两种对映体形式。它被广泛用于合成特殊的手性配体/催化剂,例如 TADDOL,并作为合成过多化合物的起点。几种新的 C-C 键形成反应的出现为开发基于手性池化合物的新型合成策略提供了机会。我们发现从酒石酸衍生的双二甲基酰胺/Weinreb 酰胺的去对称化可以通过控制添加格氏试剂/有机锂试剂来实现,导致单酮酰胺,其还原得到 γ-羟基酰胺。
更新日期:2021-08-16
中文翻译:

酒石酸中的 γ-羟基酰胺:用于天然产物全合成的多功能手性构件
“手性池”化合物具有明确定义的立体中心和合适的功能,是合成天然产物和具有治疗意义的化合物的极好基石。酒石酸是一种 C 2-对称分子有两种对映体形式。它被广泛用于合成特殊的手性配体/催化剂,例如 TADDOL,并作为合成过多化合物的起点。几种新的 C-C 键形成反应的出现为开发基于手性池化合物的新型合成策略提供了机会。我们发现从酒石酸衍生的双二甲基酰胺/Weinreb 酰胺的去对称化可以通过控制添加格氏试剂/有机锂试剂来实现,导致单酮酰胺,其还原得到 γ-羟基酰胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号